N-Cyclohexylpiperidine-1-carboxamide (7a)
Melting point: 140.2 – 141.4 ºC (lit. 140 – 141 ºC)[S1]
IR (6.3 mg/mL): νmax 3460, 2937, 2856, 1640, 1510, 1451 cm-1 ;
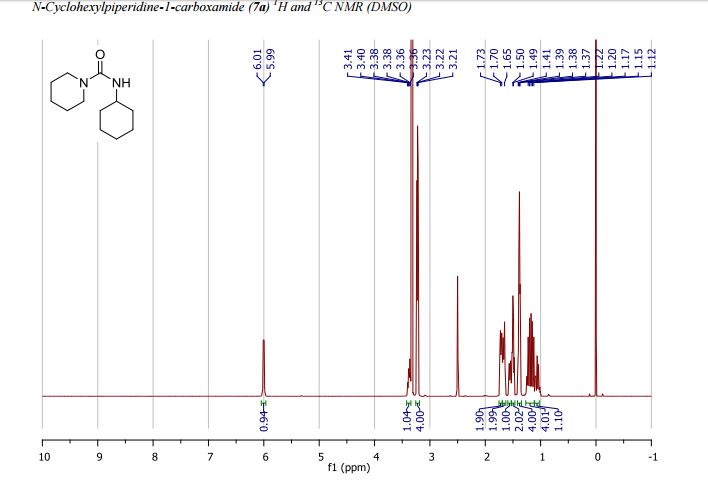
1H NMR: δ 6.00 (br d, J = 7.7 Hz, 1H), 3.37 (tdt, J = 11.0, 7.6, 3.9 Hz, 1H), 3.25 – 3.19 (m, 4H), 1.77 – 1.68 (m, 2H), 1.68 – 1.61 (m, 2H), 1.59 – 1.52 (m, 1H), 1.52 – 1.46 (m, 2H), 1.42 – 1.34 (m, 4H), 1.26 – 1.18 (m, 2H), 1.18 – 1.09 (m, 2H), 1.05 (qt, J = 12.1, 3.3 Hz, 1H) ppm;
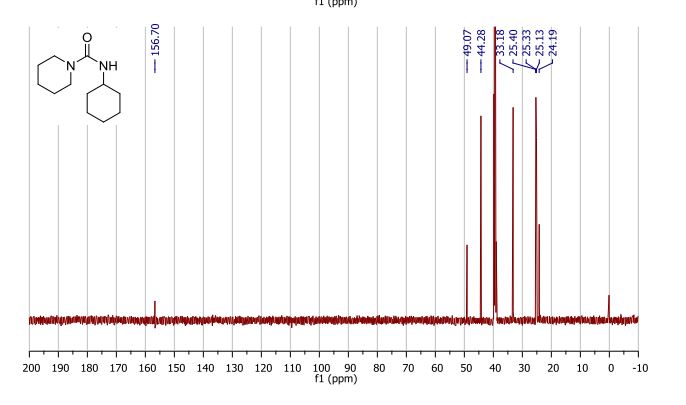
13C NMR: δ 156.7, 49.1, 44.3, 33.2, 25.4, 25.3, 25.1, 24.2 ppm;
ESI-HRMS: calcd for C12H23ON2 [M+H]+ : 211.18049; found: 211.18067; delta=0.8 ppm
Synthesis of Urea Derivatives in Two Sequential Continuous-Flow Reactors
† Department of Organic Chemistry and Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
‡ Gedeon Richter Plc., PO Box 27, 1475 Budapest, Hungary
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00019

A continuous-flow system consisting of two sequential microreactors was developed for the synthesis of nonsymmetrically substituted ureas starting from tert-butoxycarbonyl protected amines. Short reaction times could be achieved under mild conditions. In-line FT-IR analytical technique was used to monitor the reaction, including the formation of the isocyanate intermediate, thus allowing optimization of the reagent ratios. The mechanistic role of the applied base was also clarified. The setup was successfully utilized for the synthesis of several urea derivatives including the active pharmaceutical ingredient cariprazine.
References
[S1] Y. Matsumura, Y. Satoh, O. Onomura, T. Maki, J. Org. Chem. 2000, 65, 1549. doi:10.1021/jo991076k
[S2] P. Liu, Z. Wang, X. Hu, European J. Org. Chem. 2012, 2012, 1994. doi:10.1002/ejoc.201101784
////////////
