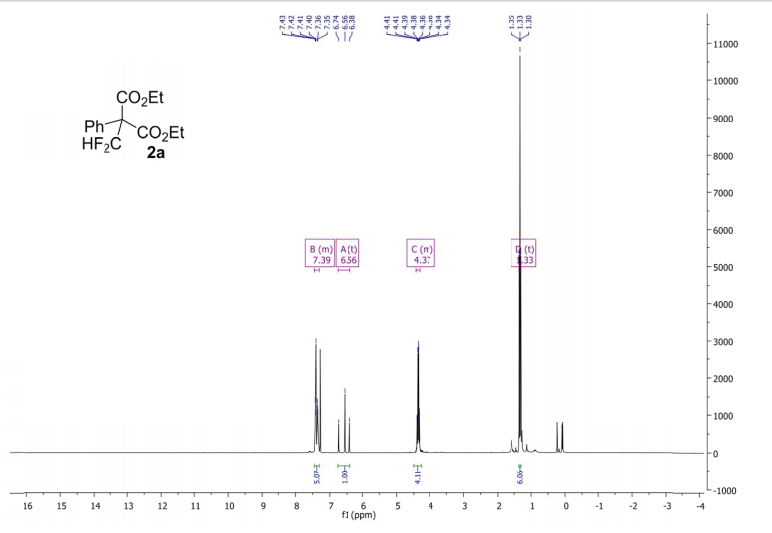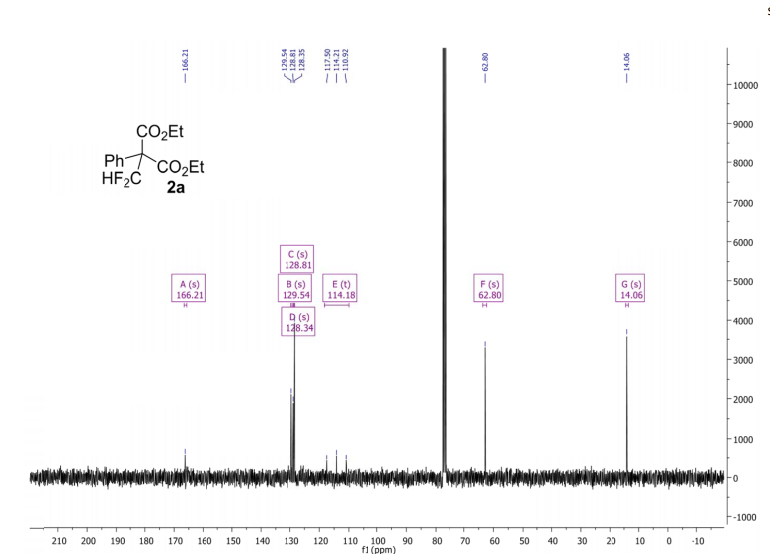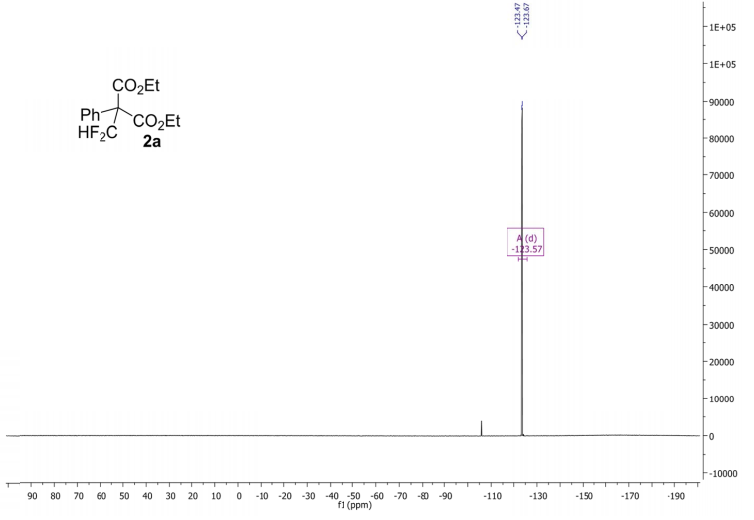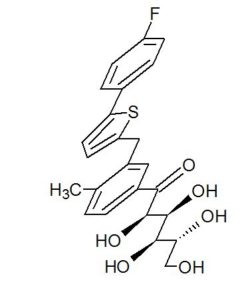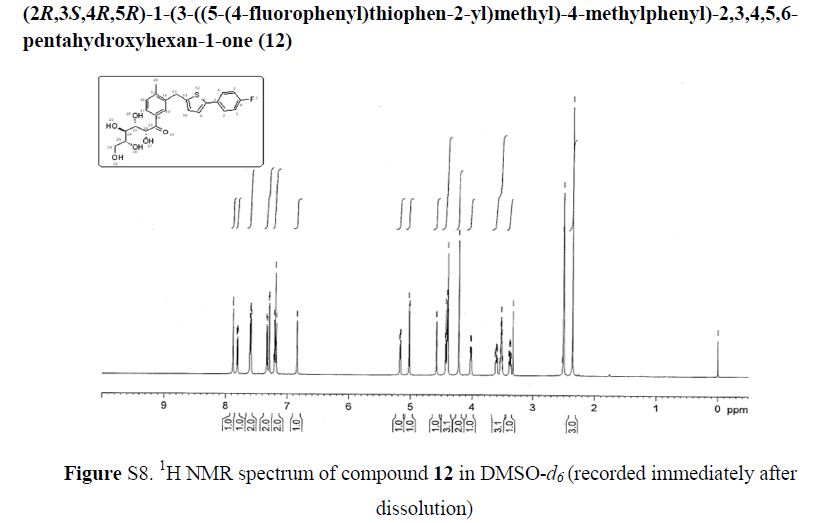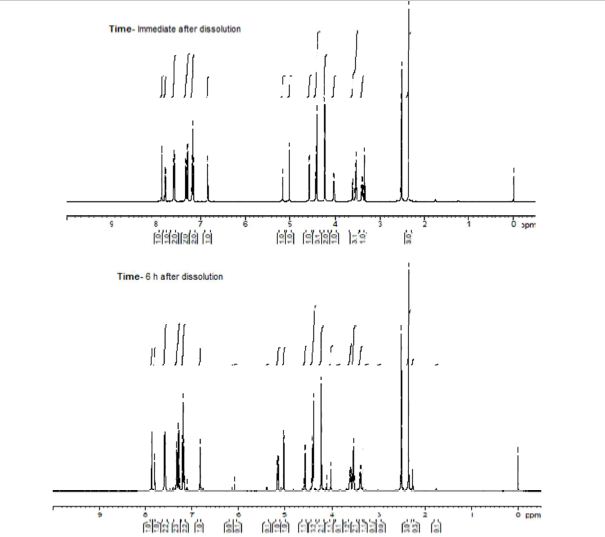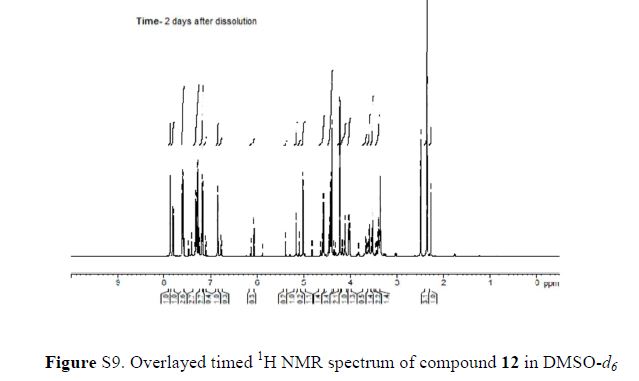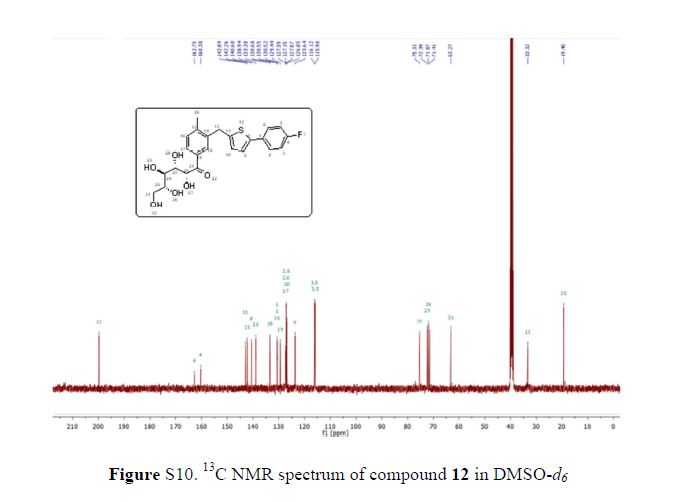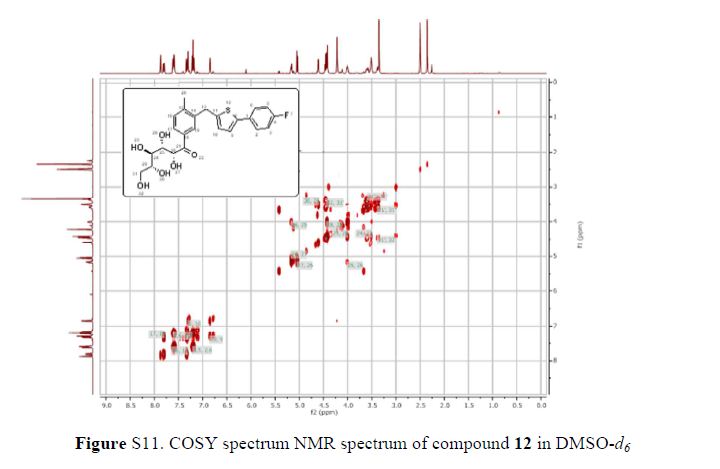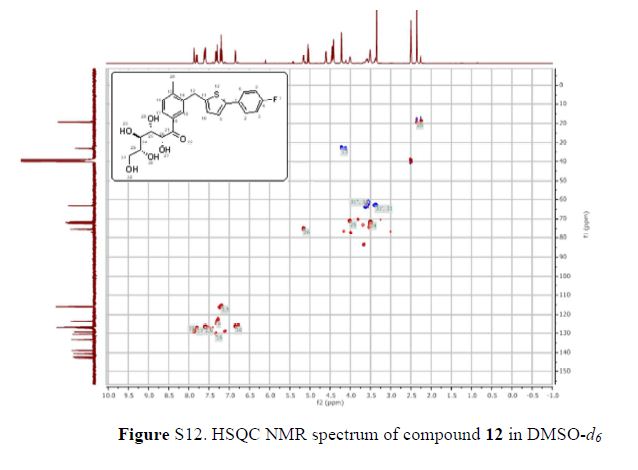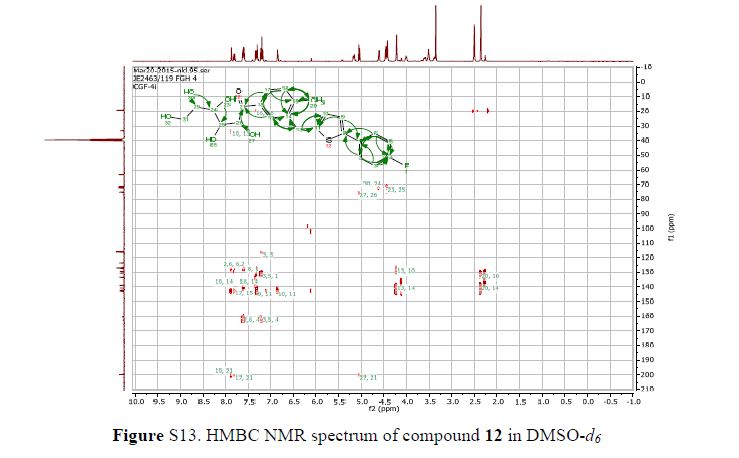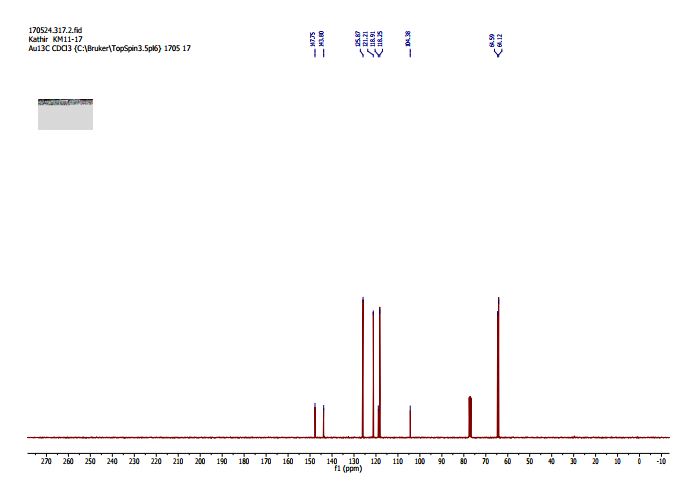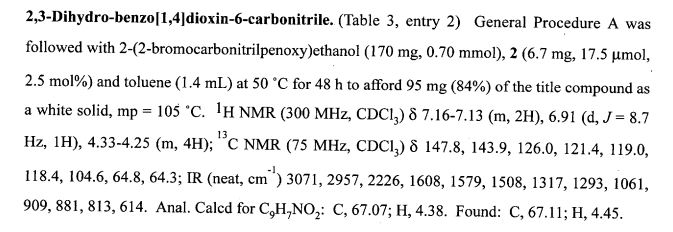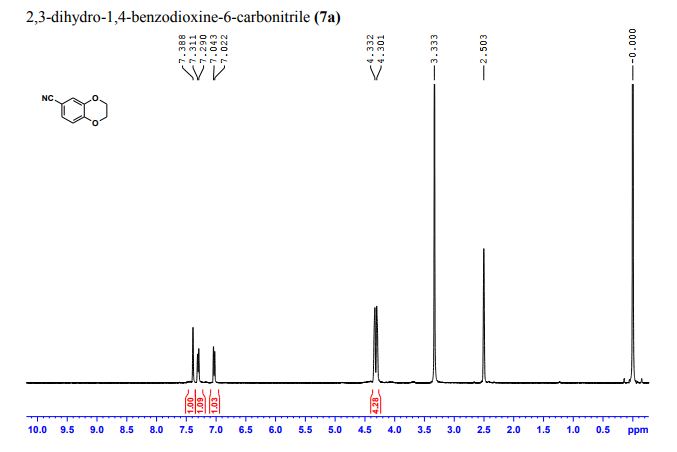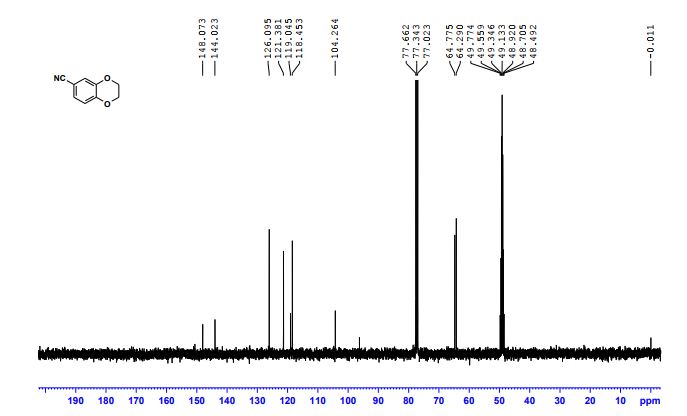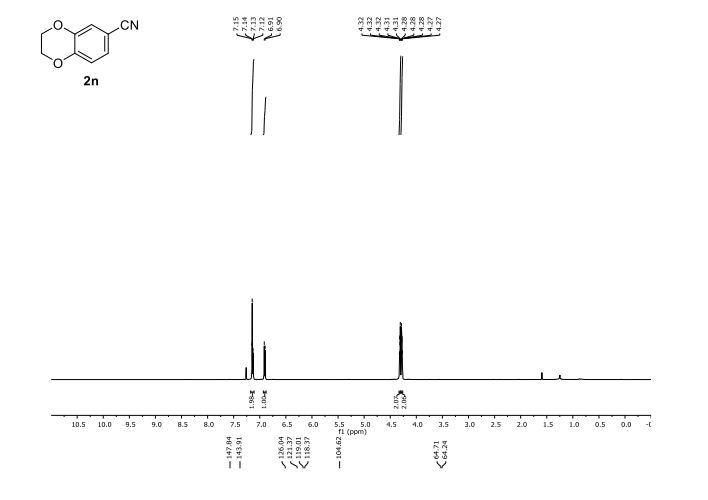
(2R,4R)-Methyl-2-tert-butyl-1,3-thiazolidine-3-formyl-4-carboxylate (18)
the resulting crude was purified by flash chromatography on silica gel (eluted by 30–50% ethyl acetate in hexane). The collected fractions were evaporated and recrystallized from diethyl ether–hexanes (1:1, v/v) to afford N-formyl thazolidine 18 (300 g, 90% yield, 97% HPLC purity) as colorless crystals.
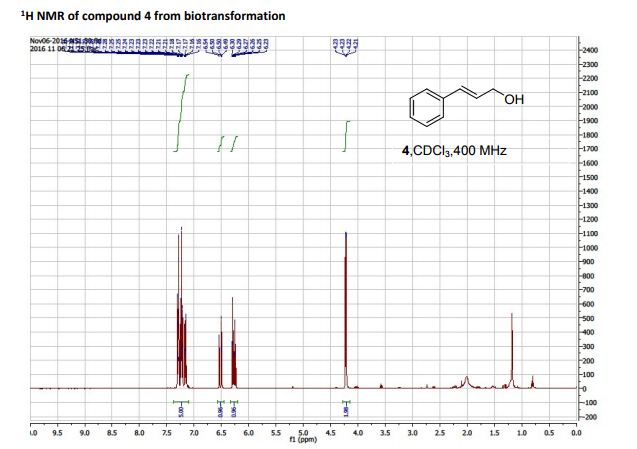
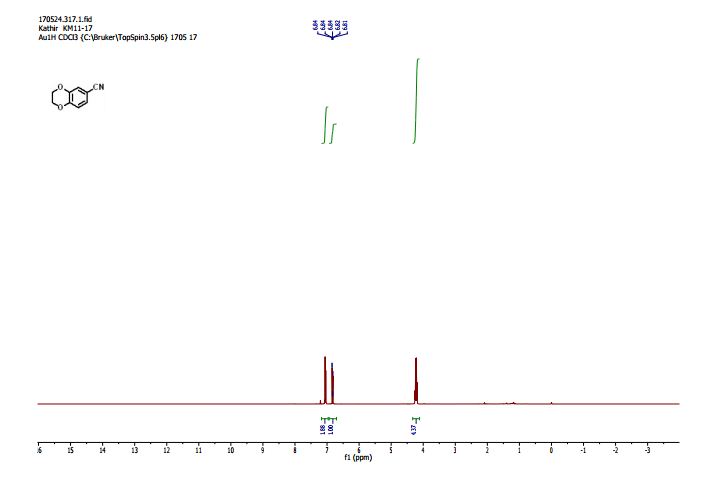
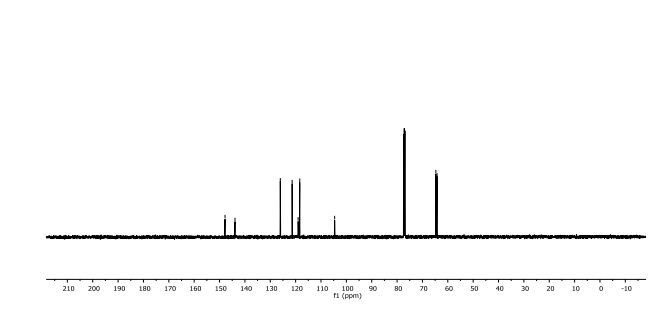
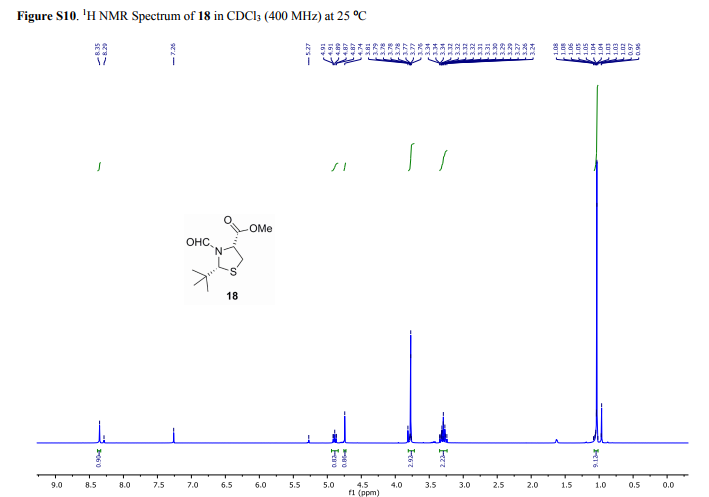
1H NMR (400 MHz, CDCl3, mixture of conformers (7:1), major): δ 8.35 (s, 1H), 4.89 (t, J = 8.0 Hz, 1H), 4.74 (s, 1H), 3.77 (s, 3H), 3.34–3.24 (m, 2H), 1.03 (s, 9H) ppm.
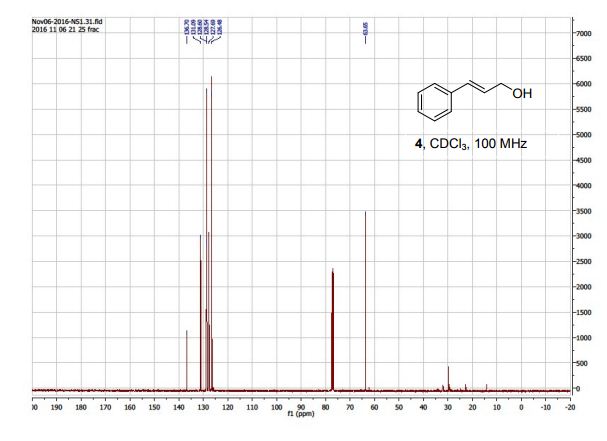
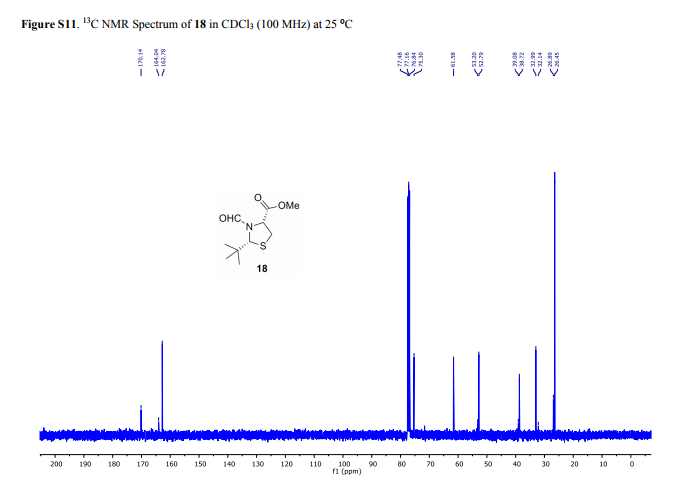
13C NMR (100 MHz, CDCl3, mixture of conformers (7:1), major): δ 170.1, 162.8, 75.3, 61.6, 52.8, 38.7, 33.0, 26.5 ppm. HRMS (ESI) m/z calcd for C10H17NO3S (M+H)+ 232.1002, found 232.1001.
//////////







 Open Access
Open Access