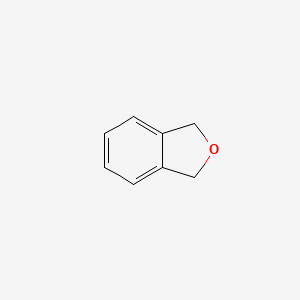
Ethyl(1R,2S,3S,4S)-2-(furan-2-yl)-3-nitro-6-oxobicyclo[2.2.2]octane-1-carboxylate
Compound 7
Ethyl(1R,2S,3S,4S)-2-(furan-2-yl)-3-nitro-6-oxobicyclo[2.2.2]octane-1-carboxylate
To a solution of CAT 10 (128 mg, 0.37 mmol) and the nitroolefin 9 (3.1 g, 22.3
mmol) in 10 mL anhydrous CH2Cl2 at room temperature was added enone 8 (1.8 g,
10.7 mmol). The resulting mixture was stirred at the same temperature until enone
8 is consumed as indicated by TLC. Then DBU (0.34 mL, 3.20 mmol) was added and the mixture
was allowed to stir at ambient temperature until completion as indicated by TLC. The solution was
concentrated in vacuo and purified by flash chromatography on silica gel (Hexane / EtOAc = 20 /
1) to give 7 (2 g, 61% yield) as a yellow solid. [α]D
23 28.0 (c = 1.0, CHCl3).
1H NMR (400 MHz,
CDCl3): δ 7.29 (d, J = 0.8 Hz, 1H), 6.27 (dd, J = 2.0 Hz, J = 3.2 Hz, 1H), 6.14 (d, J = 4.0 Hz, 1H),
4.93 (m, 1H), 4.57 (d, J = 4.4 Hz, 1H), 4.11 (m, 2H), 3.04-3.02 (m, 1H), 2.80-2.75 (m, 1H), 2.60-
2.54 (m, 1H), 2.33-2.29 (m, 1H), 1.88-1.72 (m, 2H), 1.33-1.23 (m, 1H), 1.21 (t, J = 7.2 Hz, 3H).
13C NMR (100 MHz, CDCl3): δ 204.1, 168.7, 151.8, 142.5, 110.5, 108.1, 88.3, 61.3, 56.3, 42.0,
40.8, 33.7, 26.9, 19.2, 13.8.
IR (thin film): 3435, 3141, 3120, 2996, 2959, 1715, 1653, 1621, 1557,
1505, 1473, 1443, 1408, 1371, 1336, 1301, 1336, 1301, 1270, 1236, 1142, 1120, 1083, 1062, 1074,
1045, 1045, 1011, 996, 960, 930, 892, 884, 867, 803, 753, 628, 600, 508, 436 cm-1
.
LRMS (ESI):
308.0 (M+H)+
, 330.0 (M+Na)+
.
HRMS (ESI): calcd for C15H18O6N (M+H) +
: 308.1129. Found:
308.1130.
Melting point: 117-118 oC.
Concise asymmetric total synthesis of (−)-patchouli alcohol
Author affiliations
*Corresponding authors
aCAS Key Laboratory of Synthetic Chemistry of Natural Substances, Shanghai Institute of Organic Chemistry, 345 Lingling Road, Shanghai 200032, China
E-mail: bfsun@sioc.ac.cn
Abstract
The asymmetric total synthesis of (−)-patchouli alcohol was accomplished in a concise manner. Key reactions include a highly diastereo- and enantioselective formal organocatalytic [4 + 2] cycloaddition reaction, a radical denitration reaction, and an oxidative carboxylation reaction. The formal synthesis of norpatchoulenol was achieved as well.
/////////