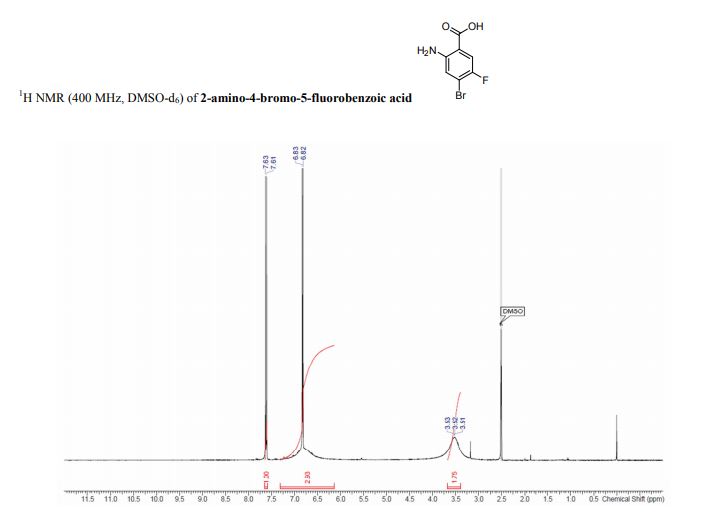
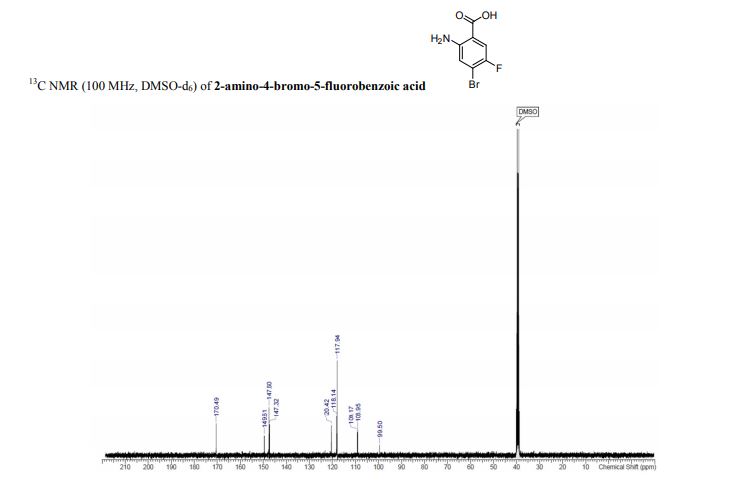
Spectral Data for 1
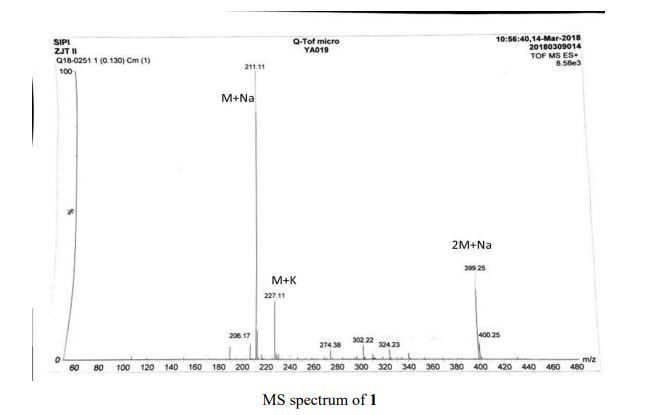
MS (ESI): calcd for [M + Na]+, 211.11; found, 211.11.
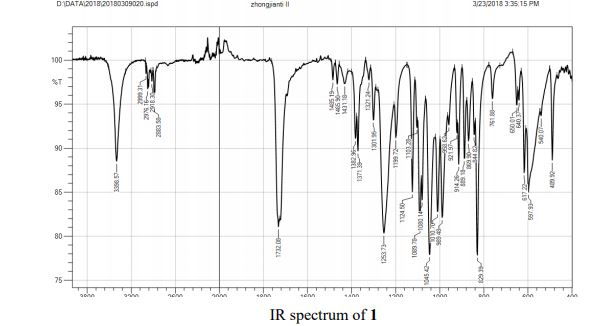
IR (KBr) cm–1: 1732.08 (C═O), 3398.57 (−OH).
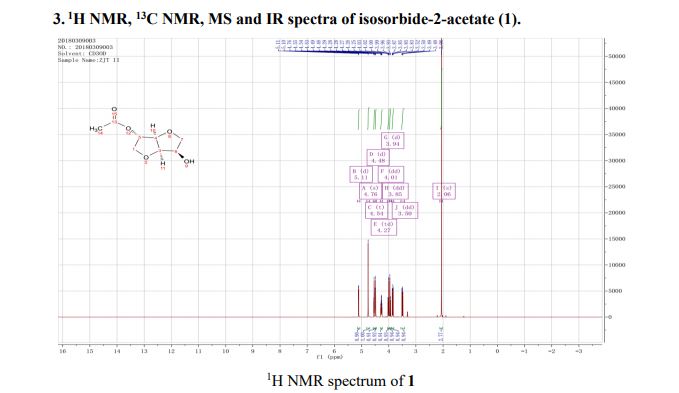
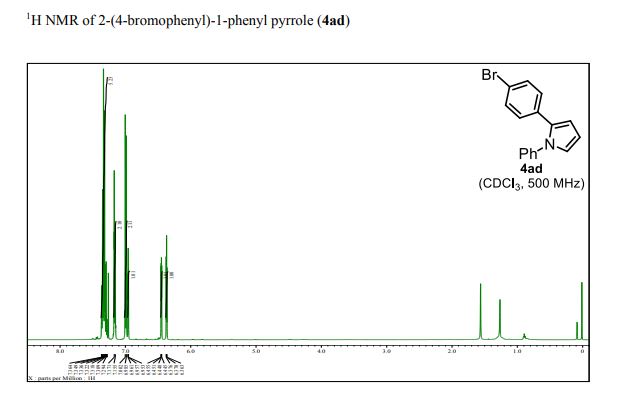
1H NMR (400 MHz, CD3OD): δ 5.11 (d, J = 3.6 Hz, 1H), 4.76 (s, 1H), 4.54 (t, J = 4.6 Hz, 1H), 4.49 (d, J = 4.4 Hz, 1H), 4.27 (td, J = 6.7, 5.1 Hz, 1H), 4.01 (dd, J = 10.7, 3.7 Hz, 1H), 3.95 (d, J = 10.7 Hz, 1H), 3.85 (dd, J = 8.8, 6.4 Hz, 1H), 3.50 (dd, J = 8.8, 7.3 Hz, 1H), 2.06 (s, 3H).
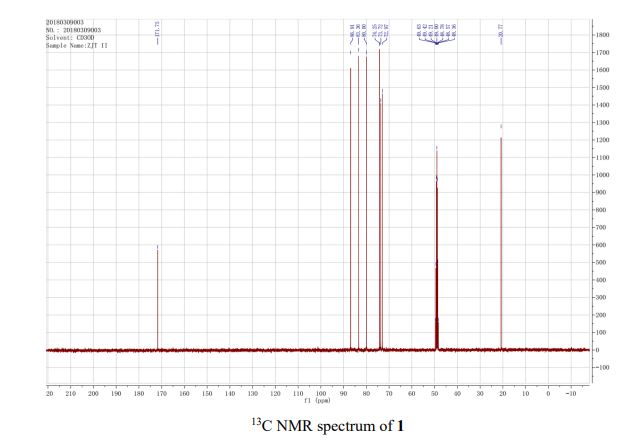
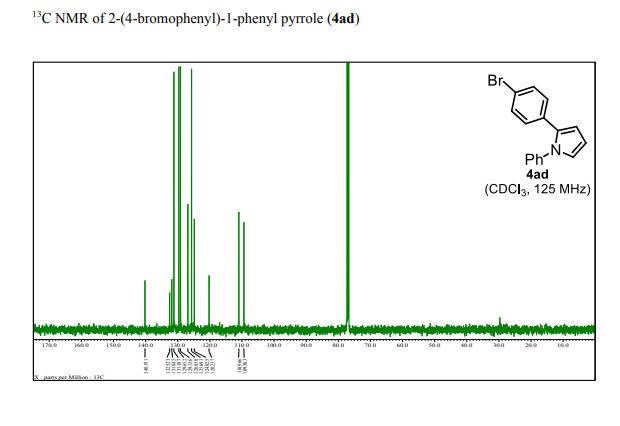
13C NMR (101 MHz, CD3OD): δ 20.8, 73.0, 73.7, 74.3, 80.0, 83.4, 86.9, 171.8.
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.8b00310
//////////////////