Synthesis of New Heterocyclic Compounds Derived From 5,10- dihydrophenophosphazine
Suad M. Al – Araji., Moayad abood Gban*
Department of Chemistry, College of Science, University of Baghdad, Baghdad, Iraq.
*Email:moayad.kban@yahoo.com
Iraqi Journal of Science, 2015, Vol 56, No.1A, pp: 12-24
http://www.iasj.net/iasj?func=fulltext&aId=99208

Iraqi Journal of Science
المجلة العراقية للعلوم
Abstract:
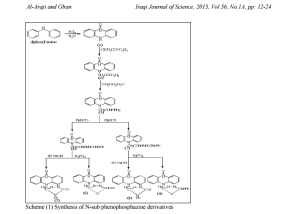
This work comprises the synthesis of 18 new N- substituted 5,10- dihydrophenophosphazine.The diphenylamine was chosen as the starting material , which was reacted with phosphorus trichloride at elevated temperature (200-220)0C for 6 hrs, followed by treating the reaction mixture with water to yield 5,10- dihydrophenophosphazine-10-oxide(1), this was reacted with ethylchloroacetat to obtain ethyl(5,10-dihydrophenophosphazine-10- oxide)acetate(2). Compound (2) was converted to acid hydrazide by treating with hydrazine hydrate( 98% ) to obtain 5-(5,10-dihydrophenophosphazine) acetohydrazide-10-oxide (3). The acid hydrazid was used to react with phenylisocyanat , phenylthioisocyanat to give (4,7) respectively which were used to prepare different heterocyclic compounds. Compound (5) was performed by the intramolecular cyclization of (4) in the presence of NaOH(2N).Compound (8) was synthesized by interaction of (7) with NaOH(2N).Compound (6) and (9) were obtaind upon the reaction of semicarbazide (4) and thiosemicarbazide (7) with phosphoric acid at 1200C. Compound (3) undergoes the character condensation reaction with different aromatic aldehyde in ethanol gave the shiff bases (10-18).
5-N-Ethyl (5, 10-dihydrophenophosphazine-10-oxide) acetate (2)
Amixture of 5,10-dihydrophenophosphazine-10-oxide(1),(5g,0.027mol) ethylchloroacetate (3.5ml,0.027 mol) in dry acetone (5 ml) and anhydrous K2CO3 (0.5 g) was refluxed for 24 h, then cooled ,filtered and solvent removed under reduced pressure. The resulting solid was monitored by (T.L.C) and recrystallized from ethanol.

Result and discussion
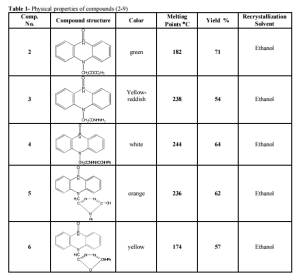
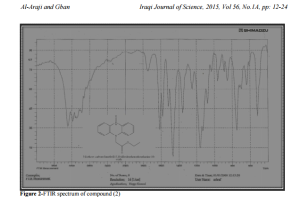
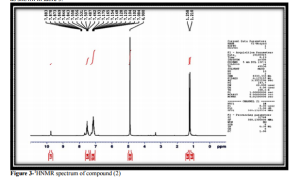
5,10-dihydrophenophosphazine-10-oxide(1) was prepared by the reaction of diphenylamine and phosphorus trichloride at 2200C followed by hydrolysis of the mixture with hot water [11] show figure 1.Compound(1) was treated with ethylchloroacetate to give N- ethyl(5,10dihydrophenophosphazine- 10-oxide)acetate(2)which was characterized by T.L.C, m.p , 1HNMR and FTIR spectrum figure 2 showed stretching bands at (3186- 3093) cm -1 for aromatic (C-H)., 2977 cm-1 for aliphatic (C-H) ., 2322 cm-1 (P-H).,1751cm-1 for ester(C=O).,(1612-1591-1520) cm-1 for (C=C) aromatic and bending band at 1465 cm-1 for (N-H) [12-14] see table2.


Iraqi Journal of Science
المجلة العراقية للعلوم



University of Baghdad, College of Science, Department of Chemistry, Karada, Baghdad,







KARADA




A view above karrada - view is taken to Baghdad's Karrada over from a military plane,
- منظر ماخوذ لبغداد من فوق الكرادة من طائرة عسكرية


///////




 .
.