4,4,5,5-tetramethyl-2-phenethyl-1,3,2-dioxaborolane
http://orgsyn.org/demo.aspx?prep=v94p0234
4,4,5,5-Tetramethyl-2-phenethyl-1,3,2-dioxaborolane (1) has the following physical and spectroscopic properties: Rf = 0.47 (3:97, ethyl acetate:pentane), the checkers report the following values for 1: Rf = 0.09 (3:97 ethyl acetate:pentane); Rf = 0.52 (10% EtOAc in hexanes); Merck silica gel 60 F254 plate; mp 38-39 °C;
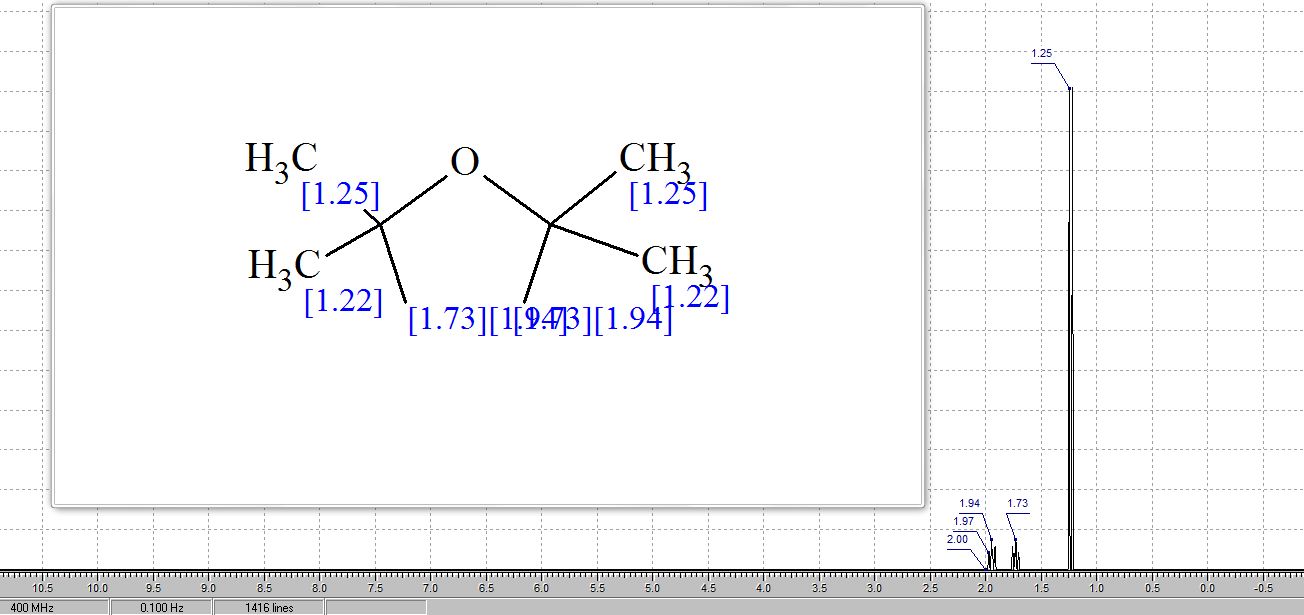
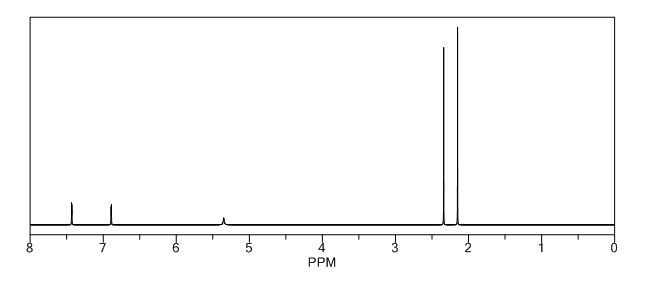
1H NMR pdf(CDCl3, 400 MHz) δ: 1.18 (t, J = 8.4 Hz, 2H), 1.26 (s, 12H), 2.79 (t, J = 8.0 Hz, 2H), 7.16-7.22 (m, 1H), 7.23-7.32 (m, 4H);
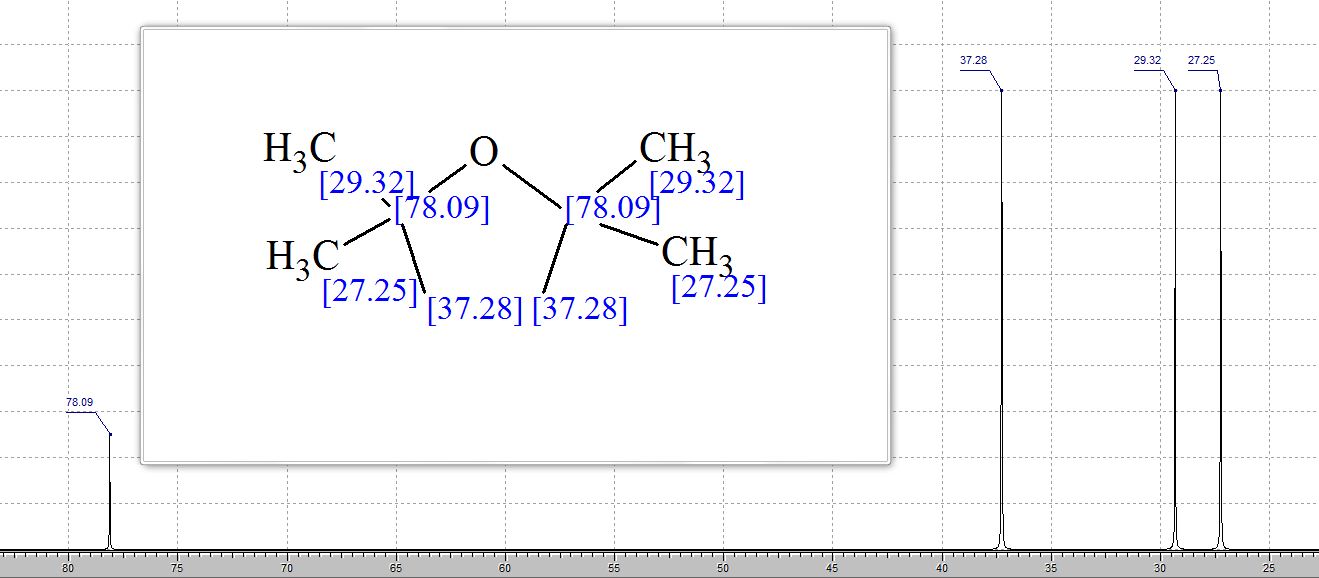
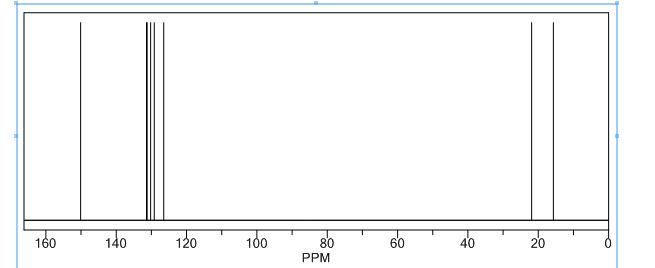
13C NMR pdf(CDCl3, 151 MHz) d: 25.0, 30.1, 83.2, 125.6, 128.1, 128.3, 144.6 [N.B. the carbon attached to boron was not observed due to quadrupolar relaxation];
HRMS (ESI+) calculated for C14H22BO2+ = 233.1707, mass found = 233.1710;
IR (film): 3026, 2978, 2929, 1372, 1318, 1139, 848, 755, 703 cm-1;
Anal. calcd for C14H21BO2: C, 72.44; H, 9.12. Found: C, 72.18; H, 9.28.
//////////////