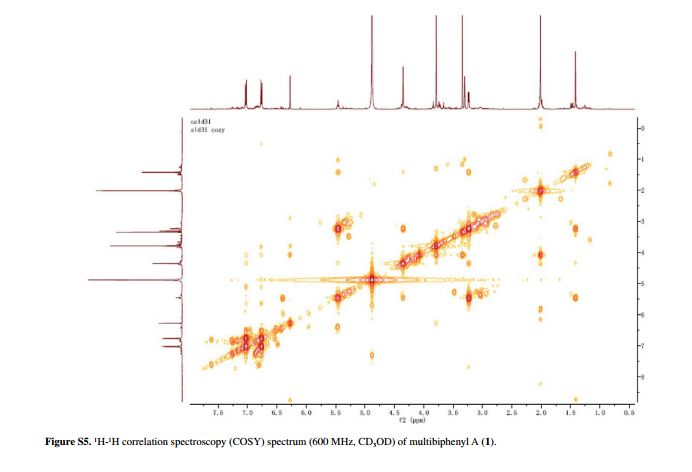

Scheme 1 General scheme of the reaction.
Scheme 2
General procedure for the synthesis of 7-chloroquinoline-1,2,3-triazoyl carboxylates
To a solution of 4-azido-7-chloroquinoline 1 (0.3 mmol, 0.061 g) in DMSO (0.3 mL), was firstly added the β-ketoesters 2a-k
(0.3 mmol) and then the catalyst pyrrolidine (0.03 mmol. 0.021 g). The
reaction mixture was stirred in an open vial at room temperature for 24
hours. After completion of the reaction, the crude product was purified
by column chromatography on silica gel using a mixture of hexanes/ethyl
acetate (5:1) as the eluent to afford the desired products 3a-k.Ethyl 1-(7-chloroquinolin-4-yl)-5-methyl-1H-1,2,3-triazole-4-carboxylate (3a)
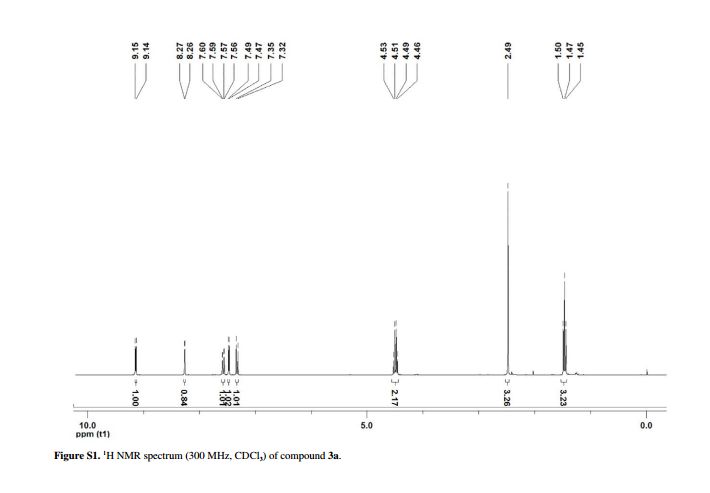
Yield: 0.085 g (90%); white solid; mp 128-130 °C;1H NMR (CDCl3, 300 MHz) δ 9.15 (d, 1H, J4.5 Hz, HetAr-H), 8.27 (d, 1H, J1.9 Hz, HetAr-H), 7.60 (dd, 1H, J9.0 and 1.9 Hz, HetAr-H), 7.48 (d, 1H, J4.5 Hz, HetAr-H), 7.34 (d, 1H, J9.0 Hz, HetAr-H), 4.50 (qua, 2H, J7.1 Hz, OCH2), 2.49 (s, 3H, CH3), 1.47 (t, 3H, J7.1 Hz, CH3);
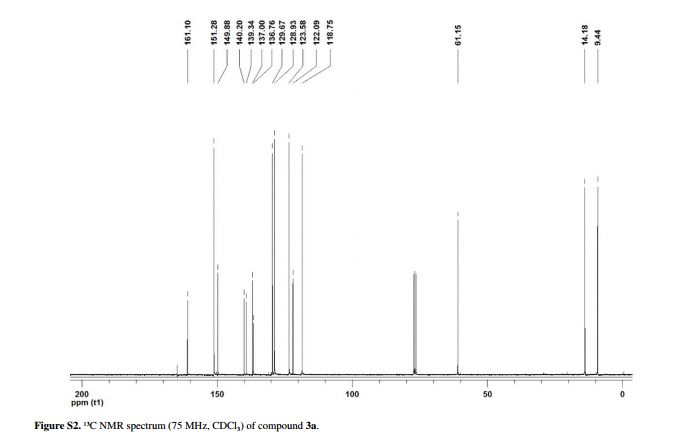
13C NMR (CDCl3, 75 MHz) δ 161.10, 151.28, 149.88, 140.20, 139.34, 137.00, 136.76, 129.67, 128.93, 123.58, 122.09, 118.75, 61.15, 14.18, 9.44;
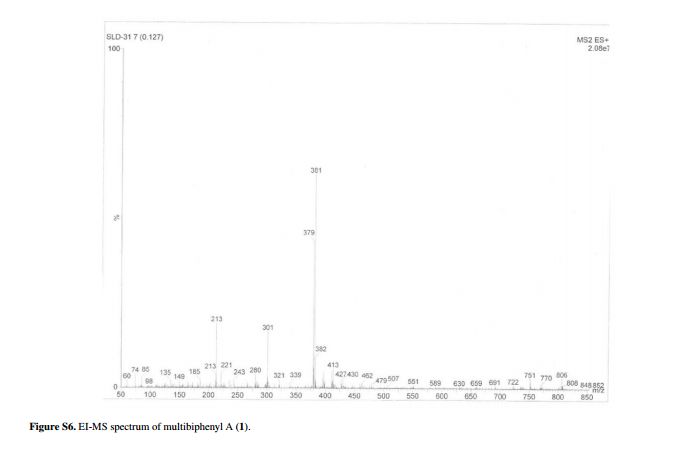
MS m/z (relative intensity): 316 (7), 259 (15), 243 (17), 231 (19), 217 (45), 215 (100), 214 (22), 205 (16), 203 (19), 189 (28), 181 (27), 179 (27), 164 (26), 162 (80), 137 (15), 135 (44), 127 (44), 126 (27), 100 (20), 99 (65), 83 (30), 75 (15), 74 (14), 43 (25);
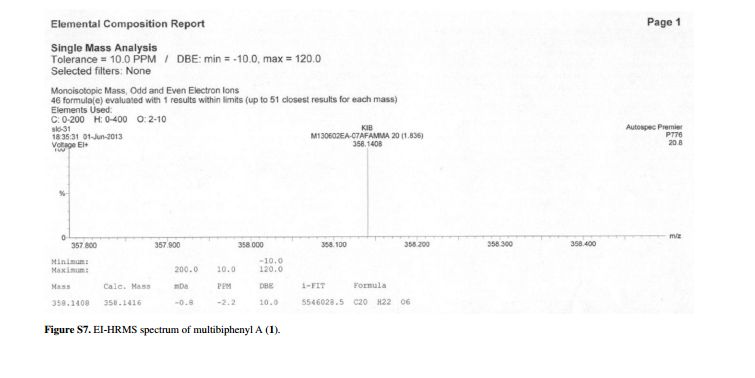
HRMS calcd. for C15H14ClN4O2 [M + H]+: 317.0805; found: 317.0788.
Journal of the Brazilian Chemical Society
On-line version ISSN 1678-4790
J. Braz. Chem. Soc. vol.27 no.1 São Paulo Jan. 2016
http://dx.doi.org/10.5935/0103-5053.20150239
ARTICLES
7-Chloroquinoline-1,2,3-triazoyl Carboxylates: Organocatalytic Synthesis and Antioxidant Properties
Maiara T. Saraivaa , Roberta Krügera , Rodolfo S. M. Baldinottib , Eder J. Lenardãoa , Cristiane Lucheseb , Lucielli Savegnagob , Ethel A. Wilhelmb * , Diego Alvesa *
aLaboratório
de Síntese Orgânica Limpa (LASOL, CCQFA), Universidade Federal de
Pelotas (UFPel), CP 354, 96010-900 Pelotas-RS, Brazil
bGrupo
de Pesquisa em Neurobiotecnologia (GPN), CDTec/CCQFA, Universidade
Federal de Pelotas (UFPel), CP 354, 96010-900 Pelotas-RS, Brazil
ABSTRACT
We
describe herein our results on the synthesis and antioxidant properties
of 7-chloroquinoline-1,2,3-triazoyl-4-carboxylates. This class of
compounds have been synthesized in moderated to excellent yields by the
reaction of 4-azido-7-chloroquinoline with a range of β-ketoesters in
the presence of a catalytic amount of pyrrolidine (10 mol%). The
synthesized compounds ethyl 1-(7-chloroquinolin-4-yl)-5-methyl-1H-1,2,3-triazole-4-carboxylate and ethyl 1-(7-chloroquinolin-4-yl)-5-phenyl-1H-1,2,3-triazole-4-carboxylate were screened for their in vitro
antioxidant activity and the results demonstrated that the first
compound reduces the lipid peroxidation levels induced by sodium
nitroprusside in liver of mice, while the second compound shown nitric
oxide scavenging activity. This is an efficient method to produce new
heterocyclic compounds with potential antioxidant activities.http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-50532016000100041&lng=en&nrm=iso&tlng=en
e-mail: diego.alves@ufpel.edu.br, ethelwilhelm@yahoo.com.br

Figure 1 Biologically important quinolines.
Key words: quinolines, 1,2,3-triazoles, organocatalysis, cycloaddition, antioxidant