Organic Chemists from Industry and academics to Interact on Spectroscopy Techniques for Organic Compounds ie NMR, MASS, IR, UV Etc. Starters, Learners, advanced, all alike, contains content which is basic or advanced, by Dr Anthony Melvin Crasto, Worlddrugtracker, email me ........... amcrasto@gmail.com, call +91 9323115463 India skype amcrasto64
................DR ANTHONY MELVIN CRASTO Ph.D ( ICT, Mumbai) , INDIA 25Yrs Exp. in the feld of Organic Chemistry,Working for GLENMARK GENERICS at Navi Mumbai, INDIA. Serving chemists around the world. Helping them with websites on Chemistry.Million hits on google, world acclamation from industry, academia, drug authorities for websites, blogs and educational contribution
Pages
- Home
- ABOUT ME
- DIMENSIONS IN NMR SPECTROSCOPY
- 13 C NMR
- 1H NMR
- CHEMDOODLE/INTERACTIVE SPECT PREDICT
- Animations
- HELP ME
- Multinuclear NMR Spectroscopy
- Examples of 13C NMR
- Books on NMR spectroscopy
- UV-Visible Spectroscopy
- IR SPECTRA EXAMPLES
- Journals
- Organic spectroscopy site
- Spectroscopy sites
- IR SPECTROSCOPY
- Books-2
- Recommended Web Sites for Spectra and Spectrum-rel...
- DISCLAIMER
- Mössbauer spectroscopy
- FINDING CHEMICAL SPECTRA
- Mass Spectrometry
- NMR Overview
- Characterisation of Organic Compounds
- SDBS Spectral Database System for Organic Compounds
- CHEMICAL SHIFT
- MASS SPECTROSCOPY
- Books-1
- MASSBANK PORTAL
- 11B NMR
Friday 24 June 2016
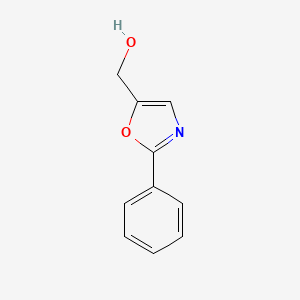
5-methylene-2-phenyl-4,5-dihydrooxazole
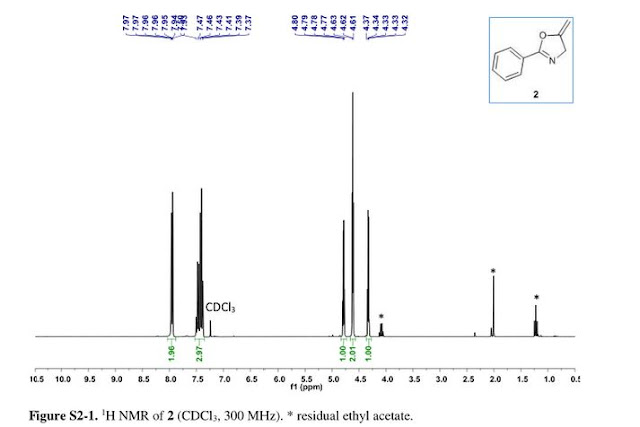
5-methylene-2-phenyl-4,5-dihydrooxazole 2:
in a dried, round-bottomed Schlenk-flask, 3.18 g (20 mmol) of propargyl amide 1 were dissolved in 60 mL of dry DCM. Then, 62 mg (0.5 mol %) of IPrAuCl and 39 mg (0.5 mol %) of AgNTf2 were added under an atmosphere of nitrogen. The reaction mixture was stirred for 24 h at room temperature. The solvent was evaporated, a small amount of PE/EA was added, and the raw product was purified by column chromatography on silica (PE/EA 15:1) to yield 2.94 g (18.4 mmol, 92%) of oxazoline 2 as a light yellow oil. Rf (PE/EA 5:1 = 0.66).
1H NMR (300 MHz, CDCl3) δ = 4.34 (q, J = 2.7 Hz, 1H), 4.63 (t,J = 2.9 Hz, 2H), 4.80 (q, J = 3.0 Hz, 1H), 7.55–7.35 (m, 3H), 8.01–7.90 (m, 2H). GC-MS (EI) m/z = 159.0 (M), 144.0 (M – CH2 – H), 131.1, 117.0, 103.0, 89.0, 77.0 (Ph).
////////////
TIBET
Tibet is a region on the Tibetan Plateau in China, Asia. It is the traditional homeland of the Tibetan people as well as some other ethnic groups such as Monpa, Qiang and Lhoba peoples and is now also ...Wikipedia




/////////////
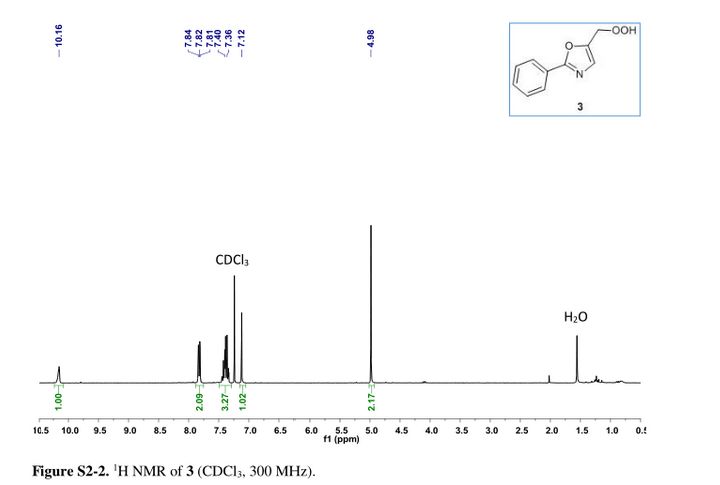
5-(hydroperoxymethyl)-2-phenyloxazole ....Safe and Fast Flow Synthesis of Functionalized Oxazoles with Molecular Oxygen in a Microstructured Reactor

The synthesis of hydroperoxymethyl oxazoles by oxidation of alkylideneoxazoles with molecular oxygen was implemented in a microstructured reactor for increased safety and larger-scale applications. Elaborate studies on the influence of pressure and temperature were performed, and the apparent activation energy for the oxidation reaction was determined. Elevated temperatures up to 100 °C and pressures up to 18 bar(a) led to a conversion rate of approximately 90% within 4 h of the reaction time, thus displaying the high potential and beneficial effect of using a microreactor setup with liquid recycle loop for this oxidation. The in situ reduction of the generated hydroperoxide functionality shows the capability of this setup for follow-up transformations.
5-(hydroperoxymethyl)-2-phenyloxazole
Oxazole–hydroperoxide 3as a colorless solid. Rf (PE/EA 3:1 = 0.31).
1H NMR (30 MHz, CDCl3) δ = 4.98 (s, 2H), 7.12 (s, 1H), 7.49–7.29 (m, 3H), 7.88–7.75 (m, 2H), 10.16 (s, 1H). GC-MS (EI) m/z = 173.1 (M – OH), 144.1 (M – CH2OOH), 116.1 (M – C6H5 + 2H), 89.1.
Safe and Fast Flow Synthesis of Functionalized Oxazoles with Molecular Oxygen in a Microstructured Reactor
† Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg,Germany
‡ Institute of Chemical Process Engineering, Mannheim University of Applied Sciences, Paul-Wittsack-Str. 10, 68163 Mannheim, Germany
§ Chemistry Department, Faculty of Science, King Abdulaziz University (KAU), 21589 Jeddah, Saudi Arabia
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.6b00118
*E-mail: t.roeder@hs-mannheim.de. Telephone: +49 621 292 6800.

Institute of Chemical Process Engineering, Mannheim University of Applied Sciences, Paul-Wittsack-Str. 10, 68163 Mannheim, Germany

Thorsten Röder
Prof. Dr.
Professor (Full)
Research experience
- Sep 2009–present
Professor (Full)
Hochschule Mannheim · Institute of Chemical Process EngineeringGermany · Mannheim - Sep 2005–Aug 2009
Laboratory Head
Novartis · Chemical and Analytical Process DevelopmentSwitzerland · Basel - Sep 1999–Aug 2004
PhD Student
Universität Paderborn · Department of Chemistry · Physical Chemistry Prof. KitzerowGermany · Paderborn
Teaching experience
- Sep 2009–present
Professor (Full)
Hochschule Mannheim · Institute of Chemical Process EngineeringGermanyLectures in: Chemical Reaction Engineering Thermodynamic Microreactors & Nanotechnology CFD Practical Course: Chemical Reaction Engineering
Education
- Oct 1999–Oct 2004
Universität Paderborn
Physical Chemistry · Dr. rer. nat.Germany · Paderborn - Sep 1994–Sep 1999
Universität Paderborn
Chemistry · Diplom ChemikerGermany

Prof. Dr. A. Stephen K. Hashmi
E-Mail hashmi@hashmi.de
/////////Safe and Fast, Flow Synthesis, Functionalized Oxazoles, Molecular Oxygen, Microstructured Reactor
TIBET FOOD
 .
.

















/////////
TIBET FOOD
 .
.


/////////
Subscribe to:
Posts (Atom)