
The synthesis of hydroperoxymethyl oxazoles by oxidation of alkylideneoxazoles with molecular oxygen was implemented in a microstructured reactor for increased safety and larger-scale applications. Elaborate studies on the influence of pressure and temperature were performed, and the apparent activation energy for the oxidation reaction was determined. Elevated temperatures up to 100 °C and pressures up to 18 bar(a) led to a conversion rate of approximately 90% within 4 h of the reaction time, thus displaying the high potential and beneficial effect of using a microreactor setup with liquid recycle loop for this oxidation. The in situ reduction of the generated hydroperoxide functionality shows the capability of this setup for follow-up transformations.
5-(hydroperoxymethyl)-2-phenyloxazole
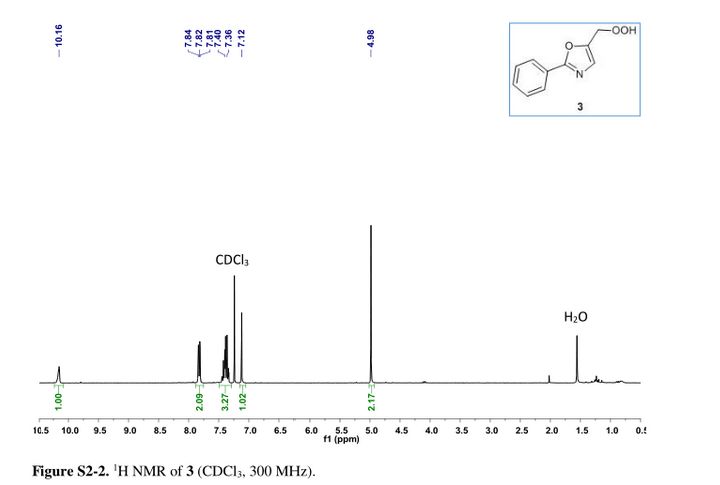
Oxazole–hydroperoxide 3as a colorless solid. Rf (PE/EA 3:1 = 0.31).
1H NMR (30 MHz, CDCl3) δ = 4.98 (s, 2H), 7.12 (s, 1H), 7.49–7.29 (m, 3H), 7.88–7.75 (m, 2H), 10.16 (s, 1H). GC-MS (EI) m/z = 173.1 (M – OH), 144.1 (M – CH2OOH), 116.1 (M – C6H5 + 2H), 89.1.
Safe and Fast Flow Synthesis of Functionalized Oxazoles with Molecular Oxygen in a Microstructured Reactor
† Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg,Germany
‡ Institute of Chemical Process Engineering, Mannheim University of Applied Sciences, Paul-Wittsack-Str. 10, 68163 Mannheim, Germany
§ Chemistry Department, Faculty of Science, King Abdulaziz University (KAU), 21589 Jeddah, Saudi Arabia
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.6b00118
*E-mail: t.roeder@hs-mannheim.de. Telephone: +49 621 292 6800.

Institute of Chemical Process Engineering, Mannheim University of Applied Sciences, Paul-Wittsack-Str. 10, 68163 Mannheim, Germany


Prof. Dr. A. Stephen K. Hashmi
E-Mail hashmi@hashmi.de
/////////Safe and Fast, Flow Synthesis, Functionalized Oxazoles, Molecular Oxygen, Microstructured Reactor
TIBET FOOD
 .
.

















/////////
TIBET FOOD
 .
.


/////////

No comments:
Post a Comment