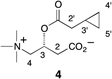
NMR Data of Cyclopropylacetyl-(R)-carnitine (4)
 | |||
|---|---|---|---|
| Position | 1H-NMR (300 MHz, D2O) HOD=4.79 | 13C-NMR (75 MHz, D2O) DSSa)=−2.04 | HMBC correlation |
| 1 | — | 177.1 | C2-Ha, Hb |
| 2 | a: 2.49 (1H, dd, J=8.0, 16.0 Hz) | 40.9 | C3-H, C4-Ha |
| b: 2.63 (1H, dd, J=5.6, 16.0 Hz) | |||
| 3 | 5.63 (1H, m) | 67.5 | C2-Ha, Hb, C4-Hb |
| 4 | a: 3.60 (1H, d, J=14.0 Hz) | 68.9 | C2-Ha, Hb, C3-H, NMe |
| b: 3.86 (1H, dd, J=9.0, 14.0 Hz) | |||
| NMe3 | 3.18 (9H, s) | 54.5 | C4-Ha, Hb |
| 1′ | — | 175.6 | C2′-Ha, Hb |
| 2′ | a: 2.27 (1H, dd, J=7.0, 16.0 Hz) | 39.7 | C4′-Ha, C5′-Ha |
| b: 2.36 (1H, dd, J=7.4, 16.0 Hz) | |||
| 3′ | 0.98 (1H, m) | 6.7 | C4′-Ha, C5′-Ha, C2′-Ha, Hb |
| 4′ | a: 0.15 (1H, m) | 4.2 | C2′-Ha, Hb |
| b: 0.52 (1H, m) | |||
| 5′ | a: 0.15 (1H, m) | 4.4 | C2′-Ha, Hb |
| b: 0.52 (1H, m) | |||
a) DSS=sodium 2,2-dimethyl-2-silapentane-5-sulfonate.
To confirm the structure of 4, the identical carnitine ester was synthesized by condensation of (R)-carnitine and cyclopropylacetic acid using an acid chloride method (see Experimental). The 1H- and 13C-NMR data of the natural and synthetic samples were identical, and the absolute configuration was also determined to be R by comparing the specific rotation of the synthetic compound and that of the natural one. Thus, the isolated cyclopropane-containing new compound was confirmed to be cyclopropylacetyl-(R)-carnitine (4). Interestingly, carnitine esters, including a cyclopropylcarboxylic acid ester, were also isolated from a Boletaceae mushroom.9) We examined the toxicity of 4 in mouse by an intraperitoneal route; however, no toxicity was detected.
Cyclopropylacetyl-(R)-carnitine is specific to genuine R. subnigricans and sufficiently stable under ordinary experimental conditions. In addition, the upfield signals in the 1H-NMR spectrum corresponding to the cyclopropane core are easily recognizable in the 1H-NMR spectrum of crude mixtures of fruiting bodies; therefore, it would be a useful chemical marker for the identification of genuine R. subnigricans (Fig. 3).
Cyclopropylacetyl-(R)-carnitine (4)
Isolation of Cyclopropylacetyl-(R)-carnitine (4) from the Russula subnigricans (Collected in Kyoto)
The fruiting bodies (500 g) of Russula subnigricans (collected in Kyoto) were cut into pieces and soaked in aqueous 0.3% AcOH (1.5 L) at 4°C overnight. The extract was filtered through filter paper under suction and then the filtrate was concentrated to about 100 mL under reduced pressure. The concentrated solution was dialyzed (relative molecular mass (Mr) 14000) against aqueous 0.3% AcOH (2.0 L×2) overnight. The dialyzate was concentrated to dryness and lyophilized to give a crude extract (27.1 g). A part (4.8 g) of the crude extract was dissolved in 1% AcOH in MeOH (48 mL), and then the soluble part was applied to an alumina column (aluminium oxide 90 standardized, Merck, 32 g), which was eluted with 1% AcOH in MeOH (300 mL). The eluate was concentrated to 5 mL, and this was diluted with aqueous 0.3% AcOH (10 mL) and chromatographed on ODS (Cosmosil 140C18 OPN, 16 g) which was eluted with H2O (300 mL) and 50% aqueous MeOH (100 mL). After removal of MeOH from the 50% MeOH fraction, the aqueous solution was washed with EtOAc (100 mL×3). The aqueous layer was concentrated in vacuo and then chromatographed on ODS by elution with 20% MeOH. The obtained fractions which contained a cyclopropane derivative were concentrated (16.2 mg) and purified by preparative TLC (ODS, 20% CH3CN) followed by HPLC (ODS ϕ6×250 mm, eluted with H2O for 10 min and then linear gradient from H2O to 20% CH3CN for 50 min at a flow rate of 1.5 mL/min with monitoring at 210 nm) to give 4 (3.4 mg, tR=20.9 min) as colorless syrup. Rf=0.31 (ODS, 1 : 4 MeOH–H2O); IR νmax (KBr): 3735, 3468, 1732, 1594, 1398, 1188, 1054 cm−1; LR-FAB-MS m/z 244 [M+H]+, 162 [M−C5H6O]+, HR-FAB-MS m/z 244.1572 [M+H]+; Calcd for C12H22NO4, 244.1549; [α]D31 −14.5 (H2O, c=0.96).
Synthesis of Cyclopropylacetyl-(R)-carnitine (4)
To a stirred cyclopropylacetic acid (0.098 mL, 1.05 mmol) was added thionyl chloride (0.080 mL, 1.10 mmol) and the mixture was stirred at room temperature (rt) for 1 h. To the crude acid chloride was directly added (R)-carnitine (86.0 mg, 0.533 mmol) and the mixture was stirred at rt for 1.5 h. After evaporation, the residue was dissolved into H2O and the aqueous layer was washed with EtOAc. The aqueous layer was applied to ODS column chromatography (H2O). The eluate was neutralized by saturated aqueous NaHCO3 solution and then concentrated to dryness. The resulting residue was extracted with MeOH and it was filtered through Celite. The filtrate and washings were concentrated in vacuo to afford cyclopropylacetyl-(R)-carnitine (4) (60.4 mg, 47% yield) as colorless foam. mp 180°C (decomp.);Rf=0.31 (ODS, 1 : 4 MeOH–H2O); IR νmax (KBr): 3735, 3433, 1734, 1592, 1392, 1179, 1034 cm−1; 1H-NMR (D2O, HOD=4.79) δ: 0.15 (2H, m, H-4′, 5′), 0.52 (2H, m, H-4′, 5′), 0.99 (1H, m, H-3′), 2.28, (1H, dd, J=7.0, 16.0 Hz, H-2′), 2.36 (1H, dd, J=7.4, 16.0 Hz, H-2′), 2.49 (1H, dd, J=8.0, 16.0 Hz, H-2), 2.63 (1H, dd, J=5.6, 16.0 Hz, H-2), 3.18 (9H, s, 4-N+Me3), 3.61 (1H, d, J=14.0 Hz, H-4), 3.86 (1H, dd, J=9.0, 14.0 Hz, H-4), 5.63 (1H, m, H-3); 13C-NMR (D2O, DSS=–2.04) δ: 4.2 (C4′, 5′), 4.4 (C4′, 5′), 6.7 (C3′), 39.7 (C2′), 40.9 (C2), 54.5 (4-N+Me3), 67.5 (C3), 68.9 (C4), 175.7 (C1′), 177.2 (C1); LR-FAB-MS m/z 244 [M+H]+, 162 [M−C5H6O]+, HR-FAB-MS m/z 244.1555 [M+H]+; Calcd for C12H22NO4, 244.1549; [α]D34 −16.6 (H2O, c=0.67).
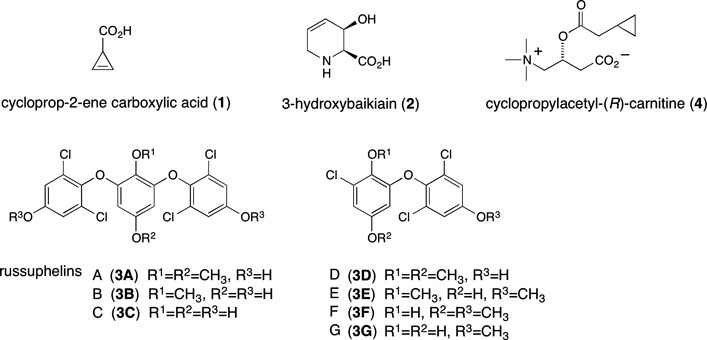
Identification of Cyclopropylacetyl-(R)-carnitine, a Unique Chemical Marker of the Fatally Toxic MushroomRussula subnigricans
Abstract
A toxic mushroom, Russula subnigricans, causes fatal poisoning by mistaken ingestion. In spite of the potent bioactivity, the responsible toxin had not been identified for about 50 years since its first documentation. Recently, we isolated an unstable toxin and determined the structure. The slow elucidation was partly due to the instability of the toxin and also due to misidentification of R. subnigricans for similar mushrooms. To discriminate genuine Russula subnigricans from similar unidentified Russula species, we searched for a unique chemical marker contained in the mushroom. Cyclopropylacetyl-(R)-carnitine specific to R. subnigricans was identified as a novel compound whose1H-NMR signals appearing in the upfield region were easily recognizable among the complicated signals of the crude extract.
/////////cyclopropylacetyl-(R)-carnitine, cycloprop-2-ene carboxylic acid, russuphelin G, mushroom poisoning, Russula subnigricans,Russulaceae
Money Island[1] or Ngwe Kyun[2] (Burmese: ငွေကျွန်း) is an island in the Mergui Archipelago of Taninthayi Region, Burma (Myanmar).
Geography
Money Island lies between Letsok-aw Island to the south and Sabi (Trotter) Island to the north. The passage between Sabi and Money Island is quite shallow.[3] The principal village on the island is Kyauk Lait, on the southeast coast. The other large village is on the northeast coast. The island is heavily forested and has small bays with sandy beaches on the west side.[3]




Money Island, Burma
Money Island, Burma
Island in Myanmar
Money Island or Ngwe Kyun is an island in the Mergui Archipelago of Taninthayi Region, Burma. Wikipedia
Geography
Money Island lies between Letsok-aw Island to the south and Sabi (Trotter) Island to the north. The passage between Sabi and Money Island is quite shallow.[3] The principal village on the island is Kyauk Lait, on the southeast coast. The other large village is on the northeast coast. The island is heavily forested and has small bays with sandy beaches on the west side.[3]
| Money Island ငွေကျွန်း |
|
|---|---|
| Island of the Mergui Archipelago | |
| Coordinates: 11°49′N 98°16′E | |
| Country | Myanmar |
| Region | Taninthayi |
| Time zone | Myanmar Standard Time (UTC+6:30) |



Map of the Mergui Archipelago
////////

No comments:
Post a Comment