(From left to right) Principal Investigator Associate Professor Gautam Sethi and NUS PhD candidate Ms Zhang Jingwen from the Department of Pharmacology at the NUS Yong Loo Lin School of Medicine led a research which found that a bioactive compound from the neem plant could significantly suppress development of prostate cancer.
Credit: National University of Singapore




Date:September 29, 2016Source:National University of SingaporeSummary:Oral administration of nimbolide, over 12 weeks shows reduction of prostate tumor size by up to 70 per cent and decrease in tumor metastasis by up to 50 per cent, report investigators.
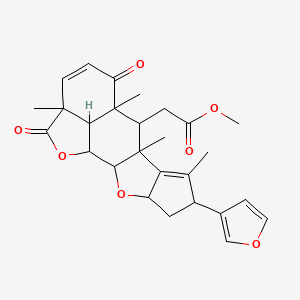
Nimbolide; NSC309909; NSC 309909; Methyl[8-(furan-3-yl)-2a,5a,6a,7-tetramethyl-2,5-dioxo-2a,5a,6,6a,8,9,9a,10a,10b,10c-decahydro-2h,5h-cyclopenta[d]naphtho[2,3-b:1,8-b'c']difuran-6-yl]acetate; CCRIS 5723;
| CAS 25990-37-8; | |
| Molecular Formula: | C27H30O7 |
|---|---|
| Molecular Weight: | 466.5229 g/mol |
Oral administration of nimbolide, over 12 weeks shows reduction of prostate tumor size by up to 70 per cent and decrease in tumor metastasis by up to 50 per cent
A team of international researchers led by Associate Professor Gautam Sethi from the Department of Pharmacology at the Yong Loo Lin School of Medicine at the National University of Singapore (NUS) has found that nimbolide, a bioactive terpenoid compound derived from Azadirachta indica or more commonly known as the neem plant, could reduce the size of prostate tumor by up to 70 per cent and suppress its spread or metastasis by half.
Prostate cancer is one of the most commonly diagnosed cancers worldwide. However, currently available therapies for metastatic prostate cancer are only marginally effective. Hence, there is a need for more novel treatment alternatives and options.
“Although the diverse anti-cancer effects of nimbolide have been reported in different cancer types, its potential effects on prostate cancer initiation and progression have not been demonstrated in scientific studies. In this research, we have demonstrated that nimbolide can inhibit tumor cell viability — a cellular process that directly affects the ability of a cell to proliferate, grow, divide, or repair damaged cell components — and induce programmed cell death in prostate cancer cells,” said Assoc Prof Sethi.
Nimbolide: promising effects on prostate cancer
Cell invasion and migration are key steps during tumor metastasis. The NUS-led study revealed that nimbolide can significantly suppress cell invasion and migration of prostate cancer cells, suggesting its ability to reduce tumor metastasis.

The researchers observed that upon the 12 weeks of administering nimbolide, the size of prostate cancer tumor was reduced by as much as 70 per cent and its metastasis decreased by about 50 per cent, without exhibiting any significant adverse effects.
“This is possible because a direct target of nimbolide in prostate cancer is glutathione reductase, an enzyme which is responsible for maintaining the antioxidant system that regulates the STAT3 gene in the body. The activation of the STAT3 gene has been reported to contribute to prostate tumor growth and metastasis,” explained Assoc Prof Sethi. “We have found that nimbolide can substantially inhibit STAT3 activation and thereby abrogating the growth and metastasis of prostate tumor,” he added.
The findings of the study were published in the April 2016 issue of the scientific journal Antioxidants & Redox Signaling. This work was carried out in collaboration with Professor Goh Boon Cher of Cancer Science Institute of Singapore at NUS, Professor Hui Kam Man of National Cancer Centre Singapore and Professor Ahn Kwang Seok of Kyung Hee University.
Neem — The medicinal plant
The neem plant belongs to the mahogany tree family that is originally native to India and the Indian sub-continent. It has been part of traditional Asian medicine for centuries and is typically used in Indian Ayurvedic medicine. Today, neem leaves and bark have been incorporated into many personal care products such as soaps, toothpaste, skincare and even dietary supplements.
Future Research
The team is looking to embark on a genome-wide screening or to perform a large-scale study of proteins to analyse the side-effects and determine other potential molecular targets of nimbolide. They are also keen to investigate the efficacy of combinatory regimen of nimbolide and approved drugs such as docetaxel and enzalutamide for future prostate cancer therapy.
Journal Reference:
- Jingwen Zhang, Kwang Seok Ahn, Chulwon Kim, Muthu K. Shanmugam, Kodappully Sivaraman Siveen, Frank Arfuso, Ramar Perumal Samym, Amudha Deivasigamanim, Lina Hsiu Kim Lim, Lingzhi Wang, Boon Cher Goh, Alan Prem Kumar, Kam Man Hui, Gautam Sethi. Nimbolide-Induced Oxidative Stress Abrogates STAT3 Signaling Cascade and Inhibits Tumor Growth in Transgenic Adenocarcinoma of Mouse Prostate Model. Antioxidants & Redox Signaling, 2016; 24 (11): 575 DOI:10.1089/ars.2015.6418

A PAPER
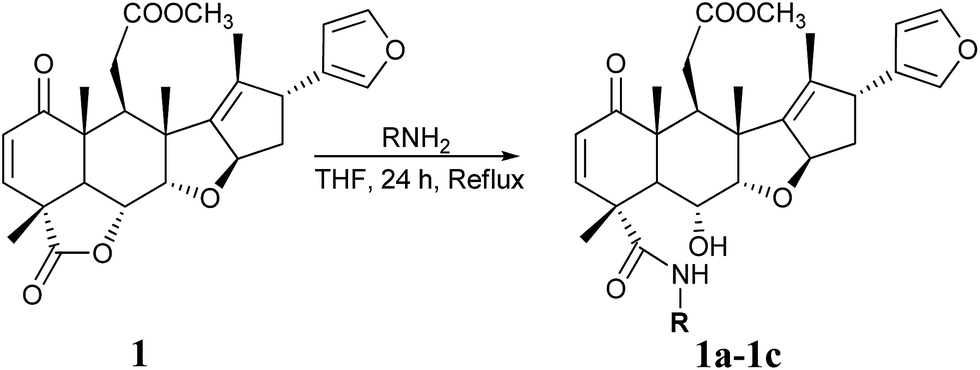
NIMBOLIDE 1
Nimbolide (1): Pale yellow crystals; C27H30O7;
FT-IR (KBr, υmax, cm -1): 2978, 1778, 1730, 1672, 1433, 1296, 1238, 1192, 1153, 1069, 951, 827, 750;
1H NMR (500 MHz, CDCl3) δH: 7.32 (t, J = 1.5 Hz, 1H), 7.28 (d, J = 9.5 Hz, 1H), 7.22 (s, 1H), 6.25 (m, 1H), 5.93 (d, J = 10.0 Hz, 1H), 5.53 (m, 1H), 4.62 (dd, J = 3.67 Hz, 12 .5 Hz, 1H), 4.27 (d, J = 3.5 Hz, 1H), 3.67 (d, J = 9.0 Hz, 1H), 3.54 (s, 3H), 3.25 (dd, J = 5.0 Hz, 16.25 Hz, 1H), 3.19 (d, J = 12.5 Hz, 1H), 2.73 (t, J = 5.5 Hz, 1H), 2.38 (dd, J = 5.5 Hz, 16.25 Hz, 1H), 2.22 (dd, J = 6.5 Hz, 12.0 Hz, 1H), 2.10 (m, 1H), 1.70 (s, 3H), 1.47 (s, 3H), 1.37 (s, 3H), 1.22 (s, 3H);
13C NMR (125 MHz, CDCl3) δC: 200.8 (CO), 175.0 (COO), 173.0 (COO), 149.6 (CH), 144.8 (C), 143.2 (CH), 138.9 (CH), 136.4 (C), 131.0 (CH), 126.5 (C), 110.3 (CH), 88.5 (CH), 82.9 (CH), 73.4 (CH), 51.8 (OCH3), 50.3 (C), 49.5 (CH), 47.7 (CH), 45.3 (C), 43.7 (C), 41.2 (CH2), 41.1 (CH), 32.1 (CH2), 18.5 (CH3), 17.2 (CH3), 15.2 (CH3), 12.9 (CH3);
HR-MS (m/z): 467.20795 [(M+H)+ ].
Content Page No 1 1H NMR spectrum of nimbolide S1 2 13C NMR spectrum of nimbolide S2 3 Mass spectrum of nimbolide
Academic Qualifications
| |||
| BSc. Chem. (Hons) | 1998 | Banaras Hindu University, Varanasi, India. | |
| MSc. Biochemistry | 2000 | Banaras Hindu University, Varanasi, India. | |
| Ph.D. Biotechnology | 2004 | Banaras Hindu University, Varanasi, India. | |
| Appointments to Date | |||
| Assistant Professor | 2008-date | Department of Pharmacology, National University of Singapore, Singapore | |
| Postdoctoral Fellow | 2004-2007 | Department of Experimental Therapeutics, The University of Texas. MD Anderson Cancer Center, Houston TX USA. | |
| Senior Research Fellow | 2002-2004 | (CSIR-NET) at School of Biotechnology, Banaras Hindu University, Varanasi, India. | |
| Junior Research Fellow | 2000-2002 | (CSIR-NET) at School of Biotechnology, Banaras Hindu University, Varanasi, India. | |
| Honours and Awards | |||
| 2007 | Ramalingaswamy fellowship from Department of Biotechnology, Government of India for outstanding research contributions in the field of Cancer Biology. | ||
| 2002 | Senior Research Fellowship award, Council of Scientific and Industrial Research, New Delhi, India. | ||
| 2000 | Junior Research Fellowship award, Council of Scientific and Industrial Research, New Delhi, India. | ||
| Research Interests | |||
| Selected Publications | |||
| Reviews and Book Chapters | |||

/////////NIMBOLIDE, CANCER, NEEM, PROSTRATE, Gautam Sethi, National University of Singapore
CC1=C2C(CC1C3=COC=C3)OC4C2(C(C5(C6C4OC(=O)C6(C=CC5=O)C)C)CC(=O)OC)C
 Dr Gautam Sethi
Dr Gautam Sethi