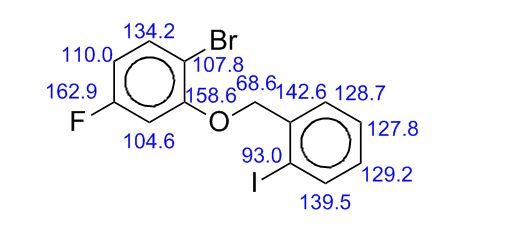
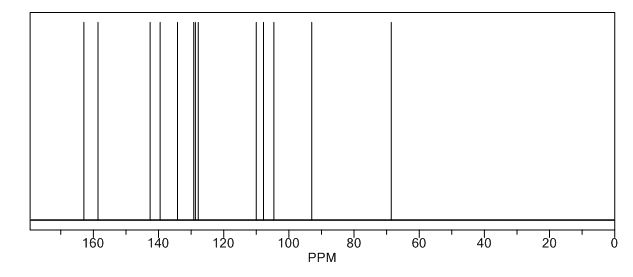
Pridopidine OXIDE
Example 5 – Preparation Of Compound 5 (4-(3-(methylsulfonyl)phenyl)-l-propylpiperidine 1-oxide)

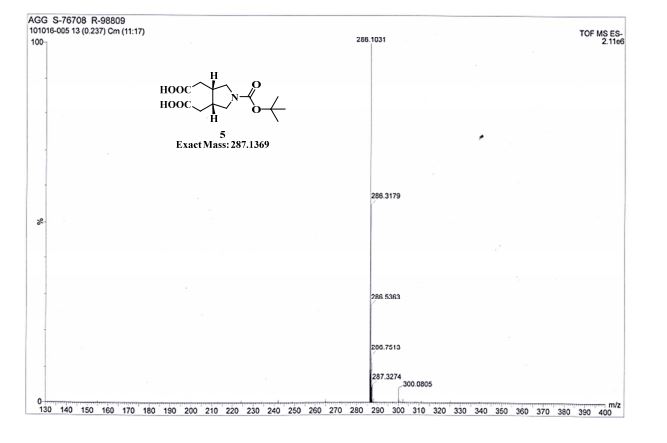
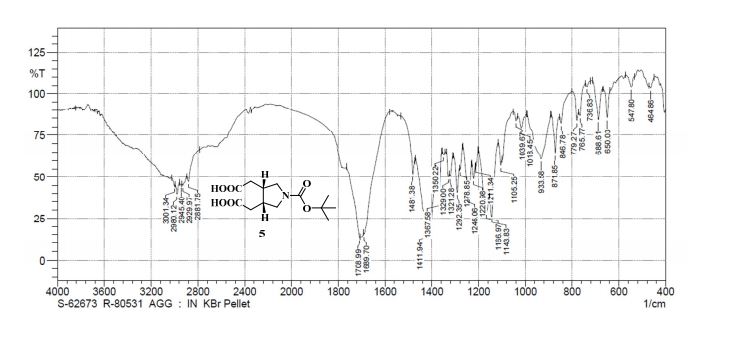
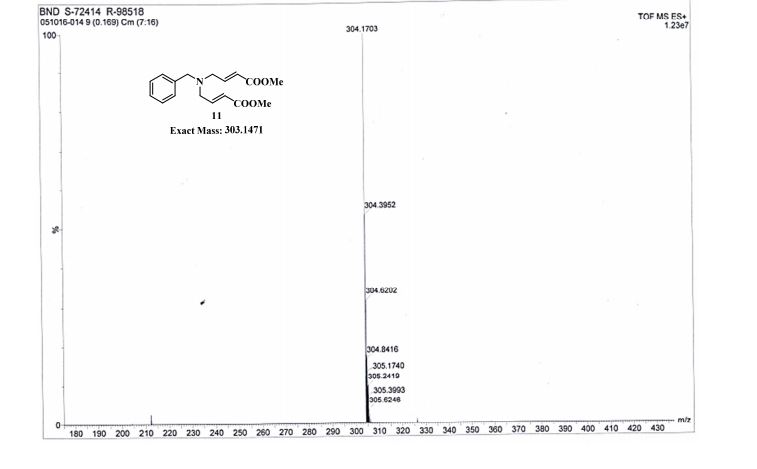
Pridopidine (50.0g, 178mmol, leq) was dissolved in methanol (250mL) and 33% hydrogen peroxide (20mL, 213mmol, 1.2eq). The reaction mixture was heated and kept at 40°C for 20h. The reaction mixture was then concentrated in a rotavapor to give 71g light-yellow oil. Water (400mL) was added and the suspension was extracted with isopropyl acetate (150mL) which after separation contains unreacted pridopidine while water phase contains 91% area of Compound 5 (HPLC). The product was then washed with dichloromethane (400mL) after adjusting the water phase pH to 9 by sodium hydroxide. After phase separation the water phase was washed again with dichloromethane (200mL) to give 100% area of Compound 5 in the water phase (HPLC). The product was then extracted from the water phase into butanol (lx400mL, 3x200ml) and the butanol phases were combined and concentrated in a rotavapor to give 80g yellow oil (HPLC: 100% area of Compound 5). The oil was washed with water (150mL) to remove salts and the water was extracted with butanol. The organic phases were combined and concentrated in a rotavapor to give 43g of white solid which was suspended in MTBE for lhr, filtered and dried to give 33g solid that was melted when standing on air. After high vacuum drying (2mbar, 60°C, 2.5h) 32.23g pure Compound 5 were obtained (HPLC: 99.5% area, 1H-NMR assay: 97.4%).
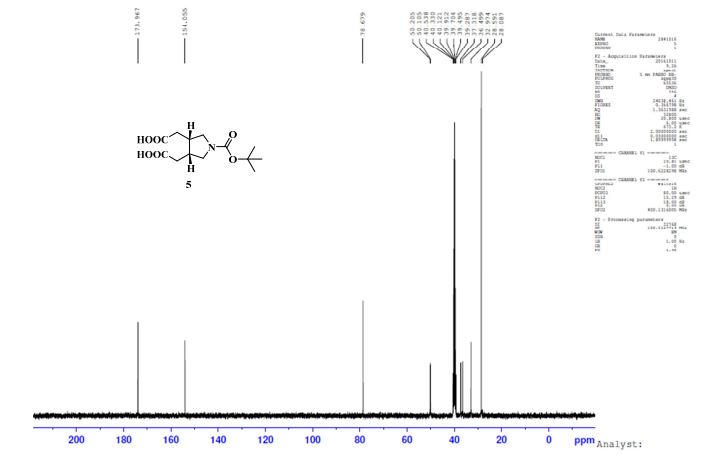
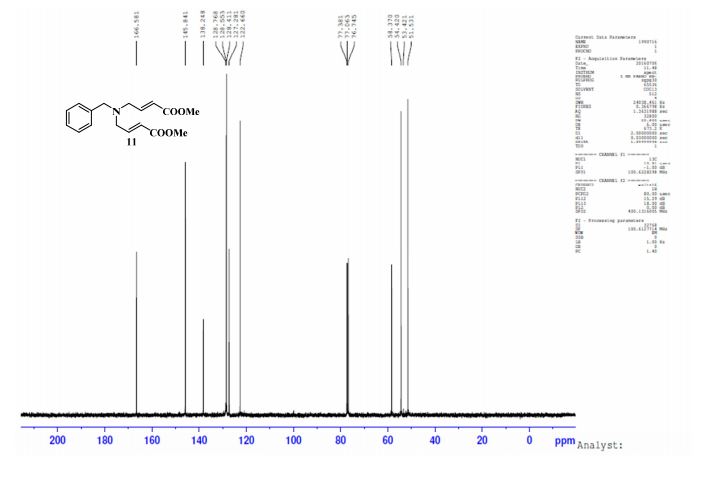
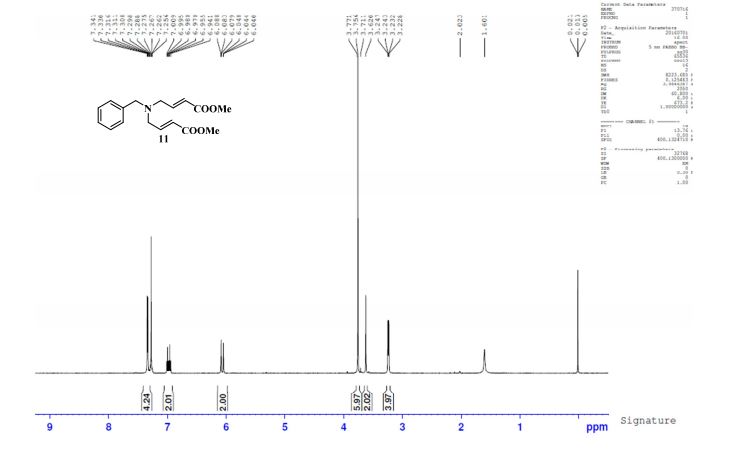
NMR Identity Analysis of Compound 5
Compound 5:
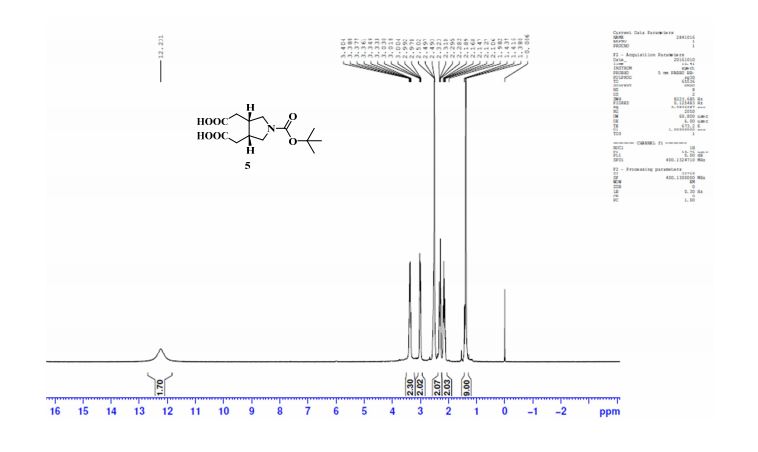
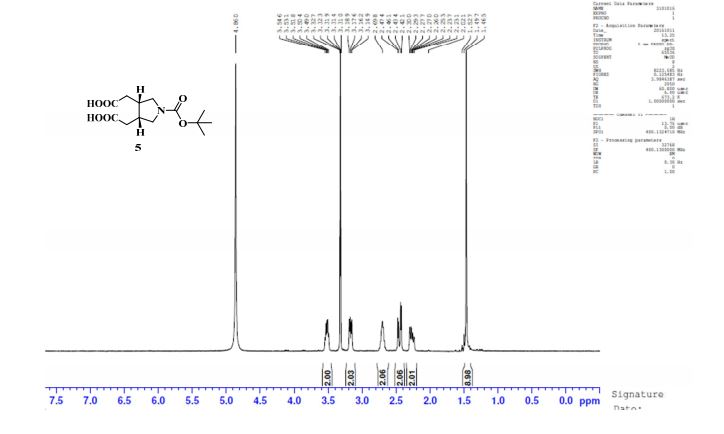
The following data in Tables 10 and 11 was determined using a sample of 63.06 mg Compound 5, a solvent of 1.2 ml DMSO-D6, 99.9 atom%D, and the instrument was a Bruker Avance ΙΠ 400 MHz.
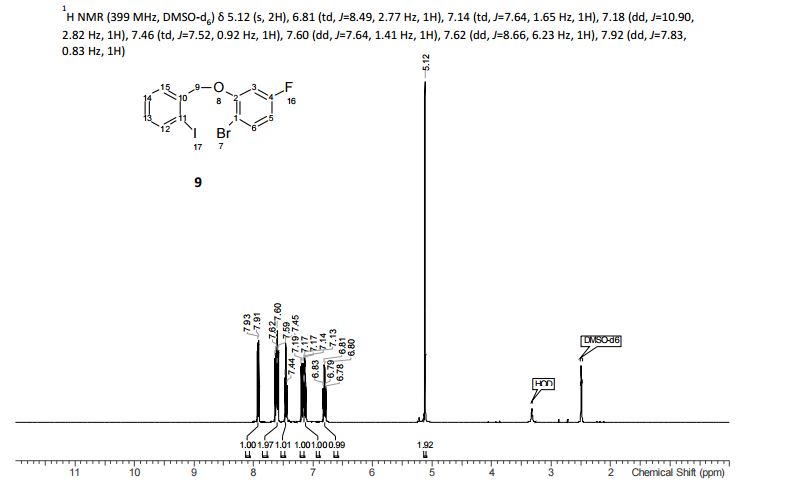
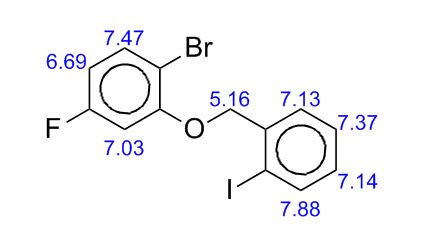
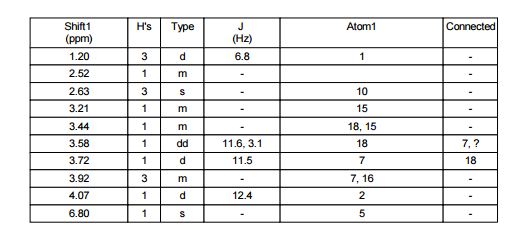
Table 10: Assignment of ¾ NMRa,c

a The assignment is based on the coupling pattern of the signals, coupling constants and chemical shifts.
b Weak signal.
c Spectra is calibrated by the solvent residual peak (2.5 ppm).
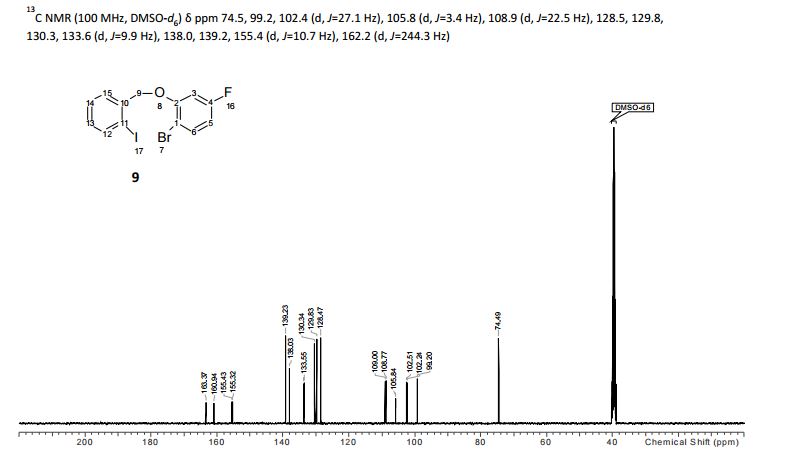
Table 11: Assignment of 13C NMRa,b

a The assignment is based on the chemical shifts and 1H-13C couplings extracted from HSQC and HMBC experiments.
b Spectra is calibrated by a solvent peak (39.54 ppm)
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016003919&recNum=5&docAn=US2015038349&queryString=EN_ALL:nmr%20AND%20PA:(teva%20pharmaceutical)&maxRec=677#H3
Example 5 – Preparation Of Compound 5 (4-(3-(methylsulfonyl)phenyl)-l-propylpiperidine 1-oxide)

Pridopidine (50.0g, 178mmol, leq) was dissolved in methanol (250mL) and 33% hydrogen peroxide (20mL, 213mmol, 1.2eq). The reaction mixture was heated and kept at 40°C for 20h. The reaction mixture was then concentrated in a rotavapor to give 71g light-yellow oil. Water (400mL) was added and the suspension was extracted with isopropyl acetate (150mL) which after separation contains unreacted pridopidine while water phase contains 91% area of Compound 5 (HPLC). The product was then washed with dichloromethane (400mL) after adjusting the water phase pH to 9 by sodium hydroxide. After phase separation the water phase was washed again with dichloromethane (200mL) to give 100% area of Compound 5 in the water phase (HPLC). The product was then extracted from the water phase into butanol (lx400mL, 3x200ml) and the butanol phases were combined and concentrated in a rotavapor to give 80g yellow oil (HPLC: 100% area of Compound 5). The oil was washed with water (150mL) to remove salts and the water was extracted with butanol. The organic phases were combined and concentrated in a rotavapor to give 43g of white solid which was suspended in MTBE for lhr, filtered and dried to give 33g solid that was melted when standing on air. After high vacuum drying (2mbar, 60°C, 2.5h) 32.23g pure Compound 5 were obtained (HPLC: 99.5% area, 1H-NMR assay: 97.4%).
NMR Identity Analysis of Compound 5
Compound 5:

The following data in Tables 10 and 11 was determined using a sample of 63.06 mg Compound 5, a solvent of 1.2 ml DMSO-D6, 99.9 atom%D, and the instrument was a Bruker Avance ΙΠ 400 MHz.
Table 10: Assignment of ¾ NMRa,c

a The assignment is based on the coupling pattern of the signals, coupling constants and chemical shifts.
b Weak signal.
c Spectra is calibrated by the solvent residual peak (2.5 ppm).
Table 11: Assignment of 13C NMRa,b

a The assignment is based on the chemical shifts and 1H-13C couplings extracted from HSQC and HMBC experiments.
b Spectra is calibrated by a solvent peak (39.54 ppm)
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016003919&recNum=5&docAn=US2015038349&queryString=EN_ALL:nmr%20AND%20PA:(teva%20pharmaceutical)&maxRec=677#H3