Catal. Sci. Technol., 2017, 7,3008-3016
DOI: 10.1039/C7CY00463J, Paper
Anastasia Rapeyko, Karen S. Arias, Maria J. Climent, Avelino Corma, Sara Iborra
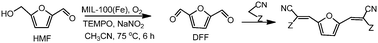
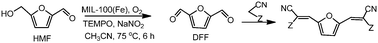
Monomers from biomass have been prepared from HMF and methylene active compounds through a one pot process using MIL-100(Fe)/TEMPO/NaNO2 as the catalytic system.
Polymers from biomass: one pot two-step synthesis of furilydenepropanenitrile derivatives with MIL-100(Fe) catalyst
*Corresponding authors
aInstituto de Tecnología Química, Universitat Politècnica de València-Consejo Superior de Investigaciones Científicas, Avenida de los Naranjos s/n, 46022 Valencia, Spain
E-mail: acorma@itq.upv.es,
siborra@itq.upv.esFax: (+34) 963877809

Anastasia Rapeyko
Química
Instituto de Tecnologia Quimica UPV-CSIC
Universitat Politècnica de València (UPV)
Valencia Area, Spain
Abstract
Furilydenepropanenitrile derivatives, which are useful as monomers, have been obtained in high yields by coupling the oxidation of 5-hydroxymethylfurfural (HMF) to 2,5-diformylfuran (DFF) and the Knoevenagel condensation of DFF with methylene active compounds in a one pot process.
The oxidation step was studied using an Fe containing metal–organic framework (MIL-100(Fe), and Fe(BTC)), a Cu containing MOF (Cu3(BTC)2), an Fe exchanged HY zeolite and homogeneous Fe salts in the presence of 2,2,6,6-tetramethylpiperidine-1-oxide (TEMPO) as a cocatalyst, NaNO2 as an additive and oxygen as the terminal oxidant.
The results showed that the synthesized MIL-100(Fe) post treated with NH4F was the most active catalyst achieving 100% HMF conversion with 100% selectivity to DFF and can be reused with good success.
Additionally, the catalytic system has been applied to the oxidation of different primary and secondary alcohols to aldehydes and ketones under mild reaction conditions with good success.
The second step, the Knoevenagel condensation of the obtained DFF with malononitrile or ethyl cyanoacetate, was performed taking advantage of the basicity of the reaction medium.



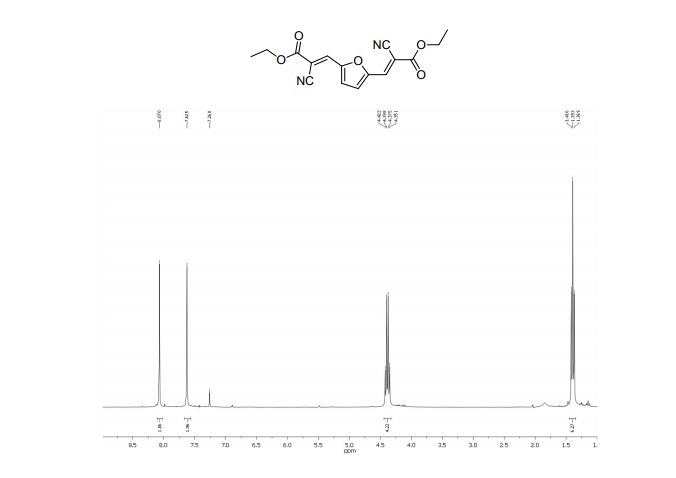
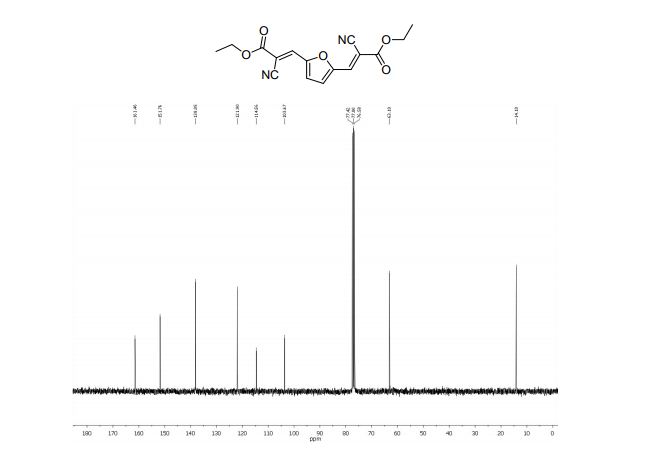
Diethyl 3,3´-(2,5-furandiyl)(2E,2’E)-bis(2-cyanoacrylate) (2b)
1H NMR (300 MHz, CDCl3): δ 8.07 (=CH, s, 2H), 7.62 (s, 2H, ArH), 4.38 (CH2, q, 4H, J = 7.1 Hz), 1.39 (CH3, t, 6H, J=7.1 Hz);
13C NMR (75 MHz, CDCl3): δ 161.4 (C=O), 151.7 (C), 138.0 (=CH), 121.9 (CN), 114.5 (CH), 103.6 (C-CN), 63.1 (O-CH2), 14.1 ppm (CH3).
MS m/z (%) 314 (M+ , 100), 286 (17), 269 (55), 240 (58), 214 (26), 196 (14), 170 (14), 142 (17), 114 (16), 89 (12), 29 (22).
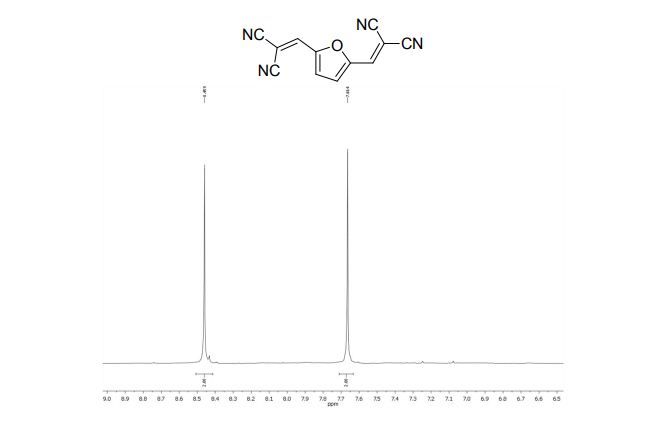
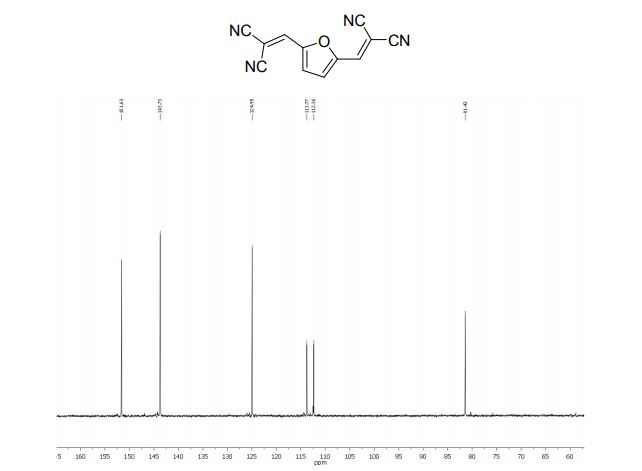
2,2´-(2,5-Furandiyldimethylidyne)-bis-propanedinitrile (2a)
1H NMR (300 MHz, DMSO-d6): δ 8.45 (=CH, s, 2H), 7.66 (s, 2H, ArH).
13C NMR (75 MHz, DMSO-d6): δ 151.6 (CH), 143.7 (C), 124.9 (CH), 113.7, 112.3, 81.4 ppm (C).
MS m/z (%) 220 (M+ , 100), 193 (9), 157 (6), 105 (15), 77 (12).
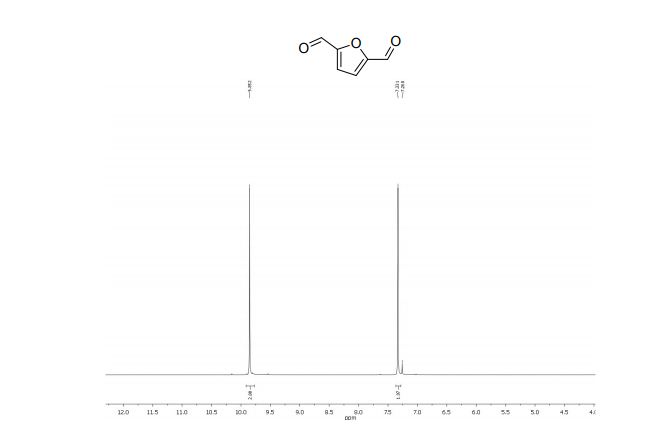
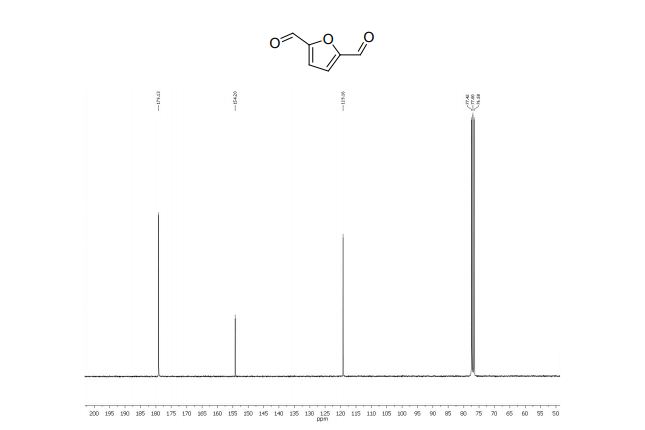
2,5-diformylfuran:
1 H NMR (300 MHz, CDCl3): δ 9.85 (s, 2H, CHO), 7.33 (s, 2H, ArH).
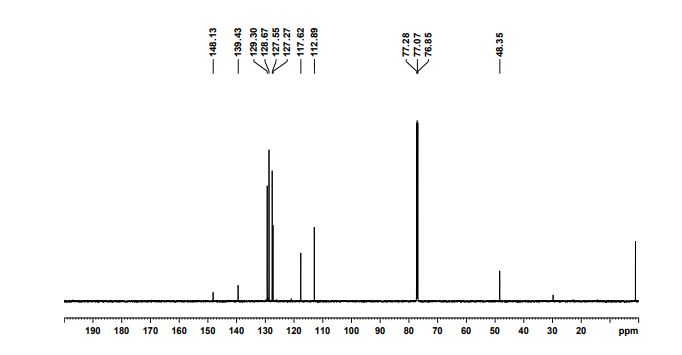
13C NMR (75 MHz, CDCl3): δ 179.1 (CHO), 154.2 (C), 119.1 ppm (CH).
MS m/z (%) 124 (M+ , 100), 123 (70), 97 (100), 95 (24), 67 (5), 39 (27), 38 (14).
Instituto de Tecnología Química, Universitat Politècnica de València-Consejo Superior de Investigaciones Científicas, Avenida de los Naranjos s/n, 46022 Valencia, Spain
///////////////









 Open Access
Open Access












.gif?id=10359&width=137)


