Catal. Sci. Technol., 2017, 7,2857-2864
DOI: 10.1039/C7CY00832E, Paper
DOI: 10.1039/C7CY00832E, Paper
Manoranjan Kumar, Vinod Bhatt, Onkar S. Nayal, Sushila Sharma, Vishal Kumar, Maheshwar S. Thakur, Neeraj Kumar, Rajaram Bal, Bikram Singh, Upendra Sharma
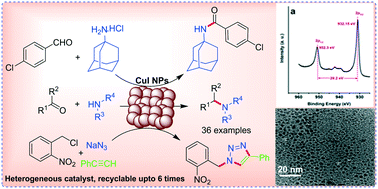
Herein, copper iodide nanoparticles (NPs) are reported for the reductive amination of carbonyl compounds for the first time.
Herein, copper iodide nanoparticles (NPs) are reported for the reductive amination of carbonyl compounds for the first time.
Catalysis Science & Technology
CuI nanoparticles as recyclable heterogeneous catalysts for C–N bond formation reactions
Abstract
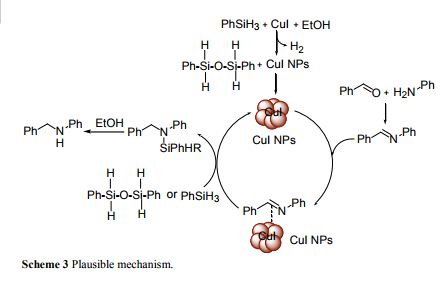
Herein, copper iodide nanoparticles (NPs) are reported for the reductive amination of carbonyl compounds for the first time. The generated NPs were characterized by TEM, EDX, XRD and XPS analyses. The XRD patterns, XPS, and EDX analysis confirmed that the resulting NPs were CuI instead of Cu. The TEM images of CuI exhibited the size of monodispersed spherical NPs in the range of 4 ± 2 nm. These generated NPs can be used as versatile heterogeneous catalysts for important organic transformations. As a proof of concept, CuI NPs were successfully applied as heterogeneous catalysts for the synthesis of secondary amines, amides and triazoles. CuI NPs can be easily recovered and recycled up to six times.
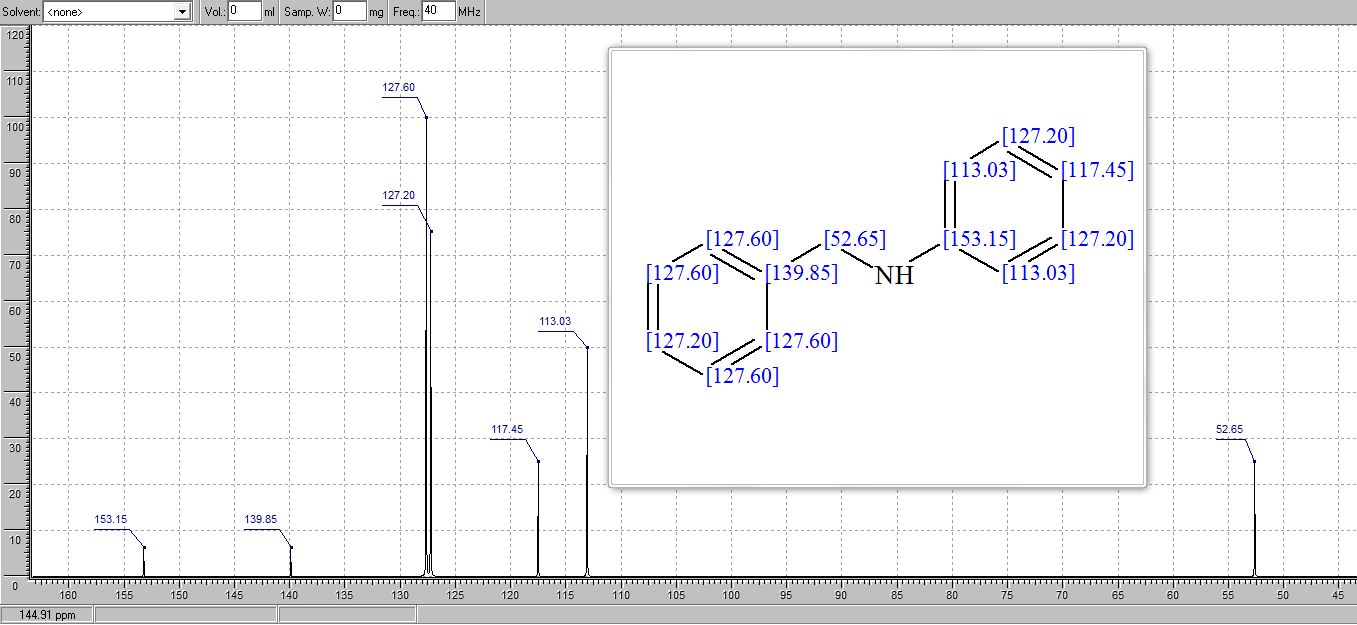
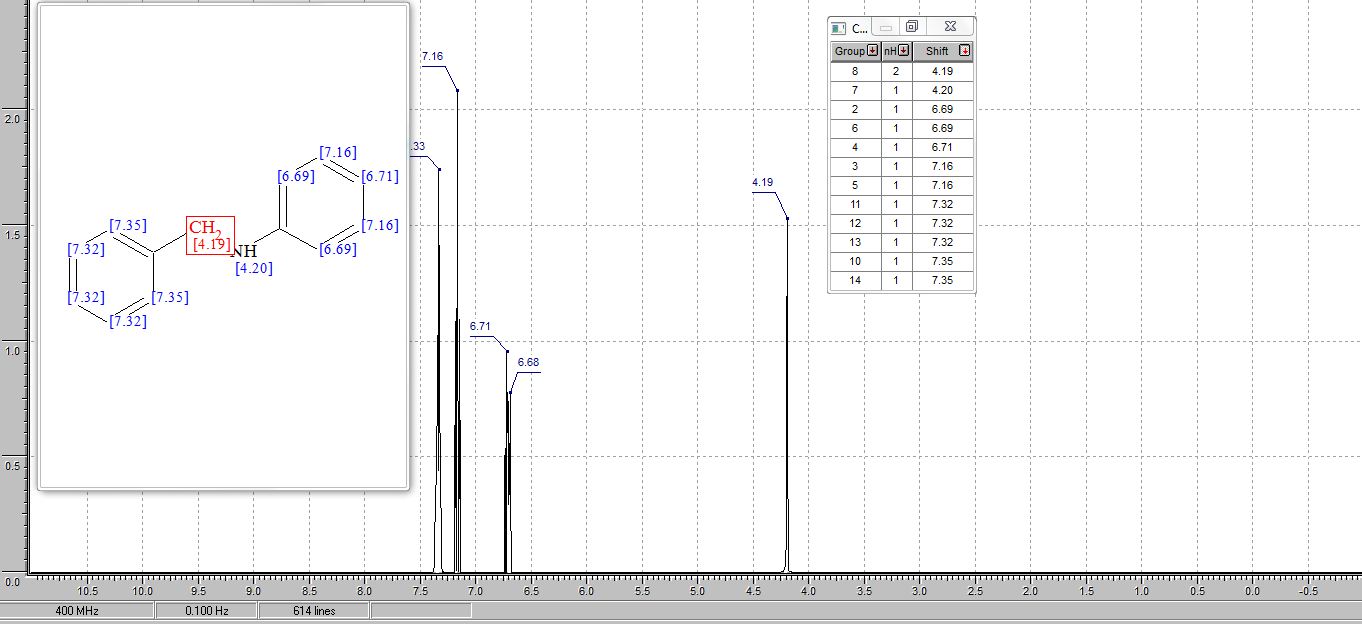
.1H and 13C NMR values of synthesized compounds
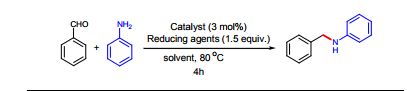
N‒benzylaniline (3a): Brown oil (168 mg, 92% yield),
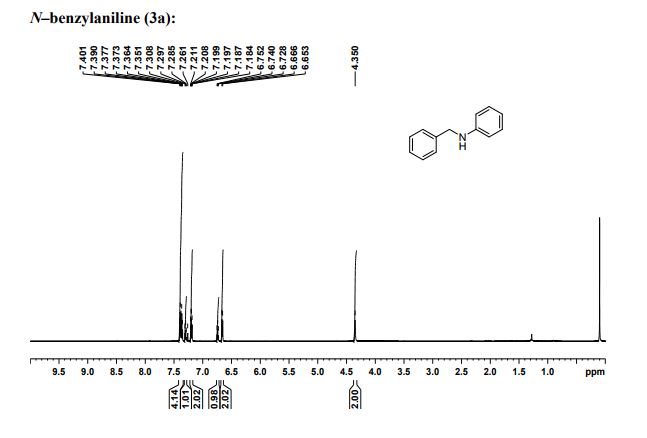
1H NMR (CDCl3, 600MHz) δ : 4.35 (s, 2H), 6.66 (d, 2H, J = 7.8 Hz), 6.72 (t, 2H, J =7.32 Hz), 7.18‒7.21 (m, 2H), 7.28 (t, 1H, J = 7.14 Hz), 7.35‒7.40 (m, 4H);
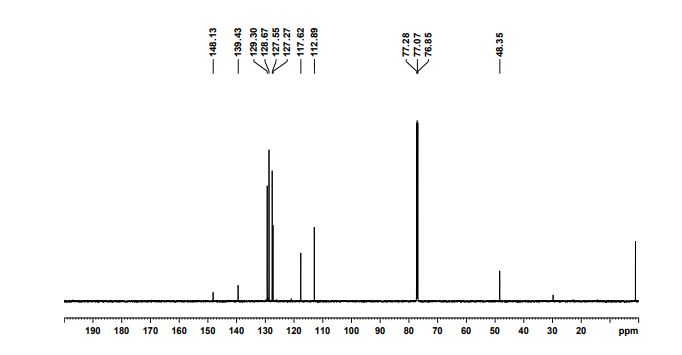
13C NMR (CDCl3, 150 MHz) δ : 48.3, 112.8, 117.6, 127.2, 127.5, 128.6, 129.3, 139.4, 148.1;
HRESIMS calcd for C13H14N [M+H]+ 184.1129, found 184.1109.
1H AND 13C NMR PREDICT
//////////

No comments:
Post a Comment