Preparation of (S)-5-Fluoro-3-methylisobenzofuran-1(3H)-one (6)
To a ................... Purity 99.9%, ee > 99.9%.

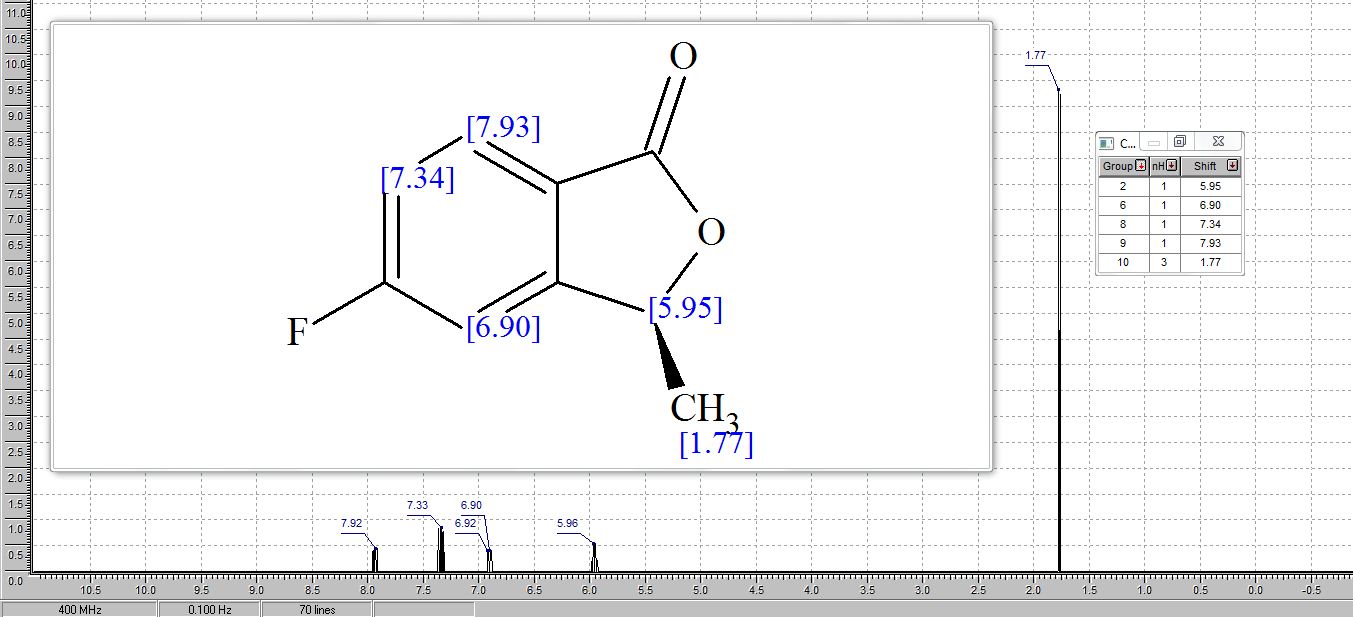
1H NMR (400 MHz, CDCl3): δ 7.88 (dd, J = 4.8, 8.4 Hz, 1 H), 7.21 (ddd, J = 2.0, 8.4, 8.8 Hz, 1 H), 7.12 (dd, J = 2.0, 8.8 Hz, 1 H), 5.53 (q, J = 6.4 Hz, 1 H), 1.63 (d, J = 6.4 Hz, 3 H) ppm;

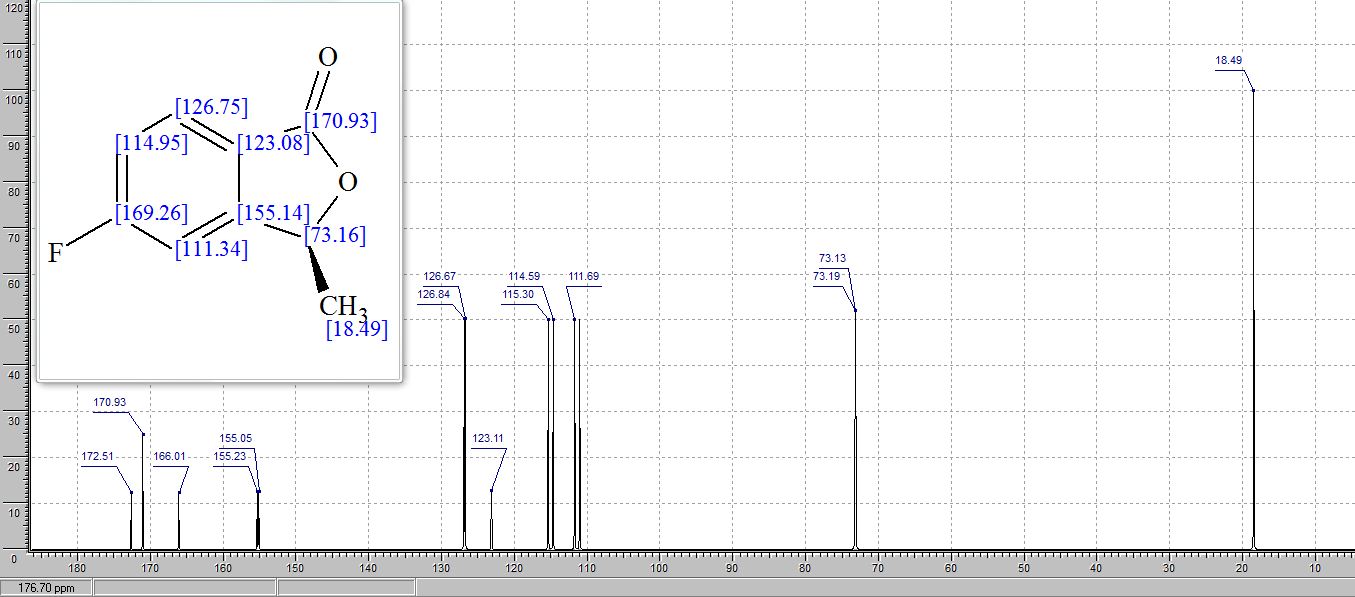
13C NMR (100.6 MHz, CDCl3): δ 169.1, 166.5 (d, JCF = 256.6 Hz), 153.8 (d, JCF = 9.1 Hz), 128.0 (d, JCF = 10 Hz), 121.8, 117.2 (d, JCF = 24.1 Hz), 108.9 (d, JCF = 25.2 Hz), 77.0, 20.2 ppm;
19F NMR (376.5 MHz, CDCl3): δ −102.8 ppm.
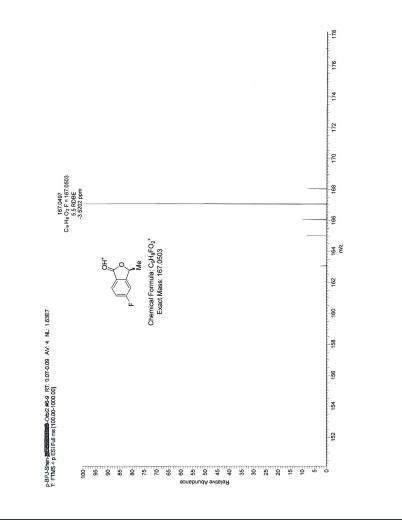
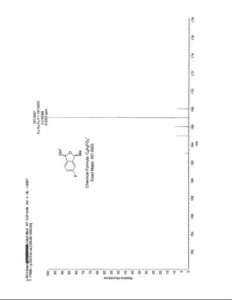
HRMS: Calcd for C9H8O2F (M + H)+: 167.0503. Found: 167.0497.
Developing an Asymmetric Transfer Hydrogenation Process for (S)-5-Fluoro-3-methylisobenzofuran-1(3H)-one, a Key Intermediate to Lorlatinib
Chemical Research and Development and Analytical Research and Development, Pfizer Worldwide Research and Development, Eastern Point Road, Groton, Connecticut 06340, United States
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00187
*E-mail: Shengquan.duan@pfizer.com.

Synthesis of (S)-5-fluoro-3-methylisobenzofuran-1(3H)-one (6), a key intermediate to lorlatinib, is described. A few synthetic methodologies, that is, boron reduction, enzymatic reduction, asymmetric hydrogenation, and asymmetric transfer hydrogenation, were evaluated for the chiral reduction of the substituted acetophenone intermediate (8). A manufacturing process, on the basis of the asymmetric transfer hydrogenation, was developed. This process was successfully scaled up to prepare 400 kg of 6.
1H AND 13C NMR PREDICT


No comments:
Post a Comment