A practical synthesis of 2,3-dihydro-1,5-benzothiazepines
Green Chem., 2017, 19,5703-5707
DOI: 10.1039/C7GC02097J, Paper
DOI: 10.1039/C7GC02097J, Paper
Domenico C. M. Albanese, Nicoletta Gaggero, Meng Fei
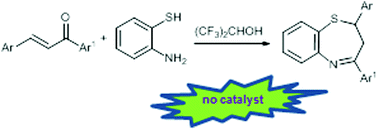
Hexafluoro-2-propanol as the solvent allows a catalyst free domino approach to 2,3-dihydro-1,5-benzothiazepines in up to 98% yield.
Hexafluoro-2-propanol as the solvent allows a catalyst free domino approach to 2,3-dihydro-1,5-benzothiazepines in up to 98% yield.
A practical synthesis of 2,3-dihydro-1,5-benzothiazepines
*Corresponding authors
LocationMilano, Italy
DepartmentDepartment of Chemistry
Positionassociate professor
Domenico Albanese received his Ph.D. degree in 1993 with Prof. Dario Landini working on phase transfer catalysis. After short stays at Imperial College London and the Technical University of Denmark, he gained a permanent position at the Università degli Studi di Milano, where he was appointed associate professor in 2008. His research interests include novel developments of phase-transfer catalysis, green chemistry and the development of new environmentally friendly antifouling agents.

Abstract
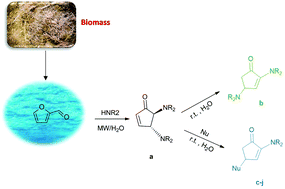
2,3-Dihydro-1,5-benzothiazepines have been obtained through a domino process involving a Michael addition of 2-aminothiophenols to chalcones, followed by in situ cyclization. Up to 98% chemical yields have been obtained at room temperature under essentially neutral conditions by using hexafluoro-2-propanol as an efficient medium.
http://pubs.rsc.org/en/Content/ArticleLanding/2017/GC/C7GC02097J?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
http://pubs.rsc.org/en/Content/ArticleLanding/2017/GC/C7GC02097J?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
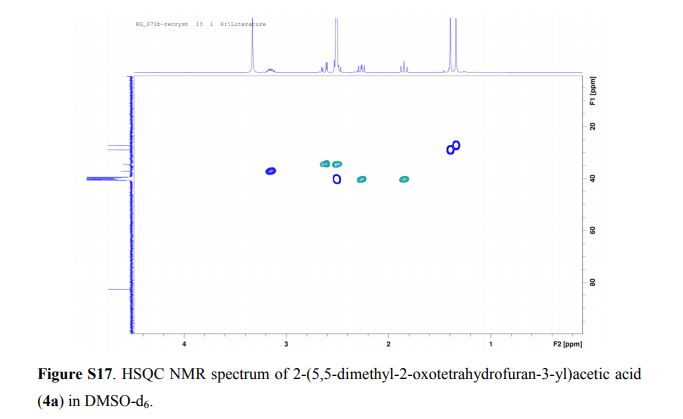
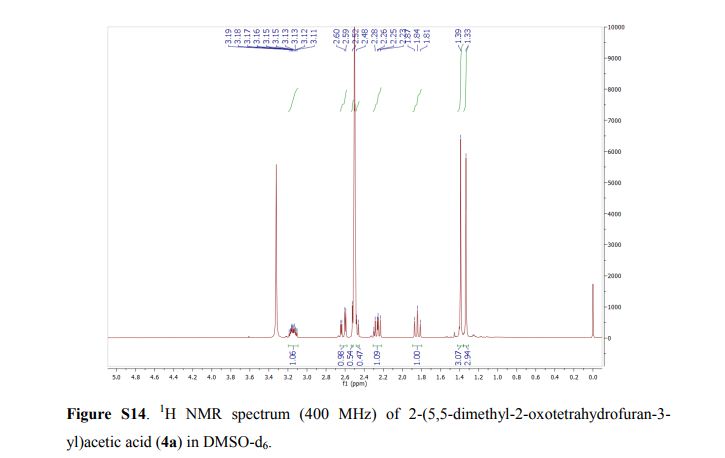
2,4-Diphenyl-2,3-dihydro-1,5-benzothiazepine (4a)
Yellow solid; mp 114-116 C [lit.1 , 114-115 °C], AcOEt/PE 1:9. 1H NMR (300 MHz, CDCl3,): 3.07 (t, J = 12.6 Hz, 1 H), 3.32 (dd, J = 4.7, 13.1 Hz, 1 H), 4.99 (dd, J = 4.5, 12.0 Hz, 1 H), 7.12-7.17 (m, 1 H), 7.25-7.30 (m, 5 H), 7.44-7.51 (m, 4 H), 7.62 (d, J = 6.1 Hz, 2 H), 8.06 (d, J = 7.5 Hz, 2 H). Isolated Yield: 339 mg, 86%.
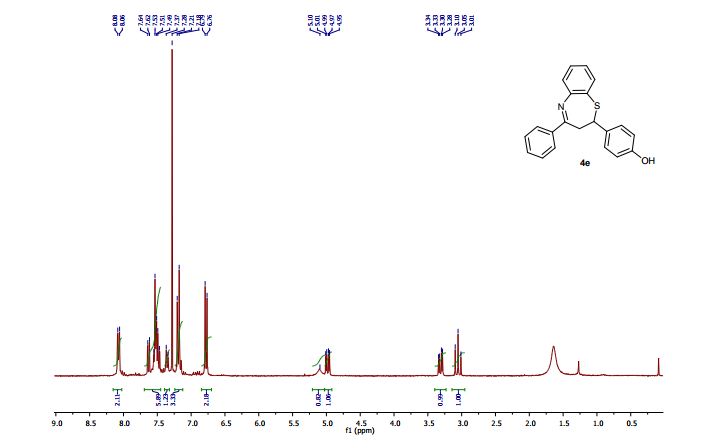
2-(4-Hydroxyphenyl)-4-phenyl-2,3-dihydro-1,5-benzothiazepine (4e)
Light brown solid; mp 131-134 °C. AcOEt/PE 40:60.
1H NMR (CDCl3, 300 MHz): = 3.01 (t, J = 12.7 Hz, 1 H), 3.28 (dd, J = 4.8, 12.9 Hz, 1 H), 4.95 (dd, J = 4.7, 12.5 Hz, 1 H), 5.10 (bs, 1 H), 6.76 (d, J = 8.5 Hz, 2 H), 7.18-7.21 (m, 3 H), 7.35 (d, J = 8.5 Hz, 1 H), 7.46- 7.55 (m, 4 H), 7.63 (dd, J =1.5, 7.7 Hz, 1 H), 8.06 (m, 2 H).
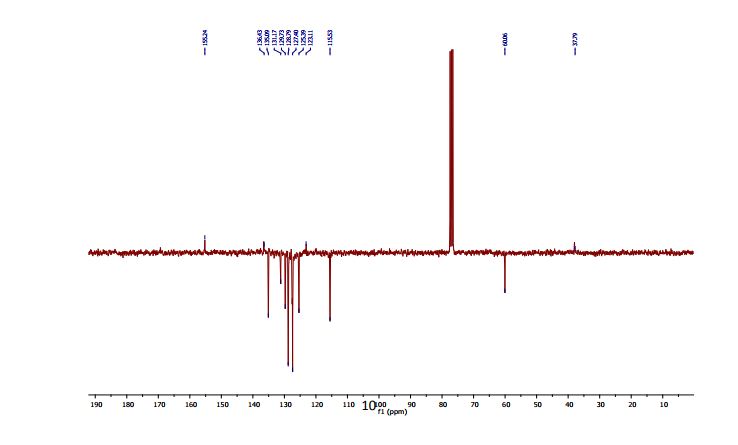
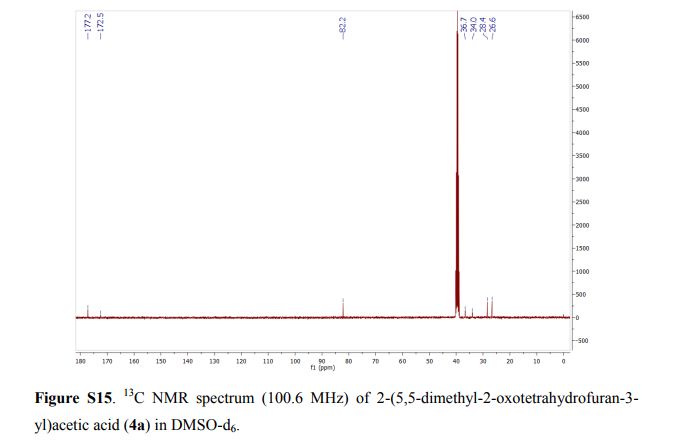
13C NMR (CDCl3, 75 MHz): 37.99 (CH2), 60.07 (CH), 115.53 (CH), 123.08 (C), 127.40 (CH), 128.79 (CH), 131.17 (CH), 136.54 (C), 141.59 (C), 155.24 (C). IR (KBr): 1599, 2921, 3350 cm-1 .
MS (ESI): m/z= 332.24 (MH)+ .
Anal. Calcd. for C21H17NOS: C, 76.10; H, 5.17; N, 4.23, found: C, 76.21; H, 5.15; N, 4.24.
Isolated Yield: 360 mg, 87%.
/////////////
'