
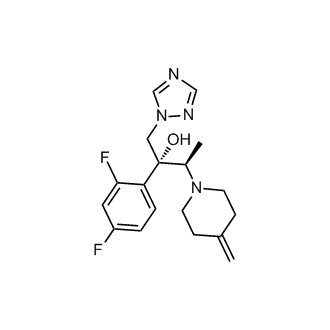
Efinaconazole
(2R,3R)-2-(2,4-Difluorophenyl)-3-(4-methylene-1-piperidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol
(2R, 3R) -2 - (2,4 - difluorophenyl) -3 - (4 - methylene-piperidin-1 - yl) -1 - (1H-1, 2,4 - triazol-1 - yl) butan-2 - manufacture ol (KP-103)
Efinaconazole is a triazole antifungal. It is approved for use in Canada as 10% topical solution for the treatment of onychomycosis (fungal infection of the nail).[1][2] Efinaconazole acts as a 14α-demethylase inhibitor.[3]
| Identifiers | |
|---|---|
| CAS number | 164650-44-6 |
| PubChem | CID 489181 |
| ChemSpider | 428538 |
| Chemical data | |
| Formula | C18H22F2N4O |
| Mol. mass | 348.39 g/mol |
PATENT
http://www.google.com/patents/WO2012029836A1?cl=en

Example 1
(2R, 3R) -2 - (2,4 - difluorophenyl) -3 - (4 - methylene-piperidin-1 - yl) -1 - (1H-1, 2,4 - triazol-1 - yl) butan-2 - manufacture ol (KP-103)
Was stirred while addition of acetonitrile 80mL, lithium hydroxide 2.859g methylene piperidine hydrobromide (4-MP · HBr) 21.26g and (119.4mmol) and (119.4mmol) - 4 obtained in Production Example 1. Then, (2R, 3S) -2 - (2,4 - difluorophenyl) -3 - methyl -2 - [(1H-1, 2,4 - triazol-1 - yl) methyl] oxirane and 20g (79.6mmol) was added, and the mixture was heated under reflux for 14 hours at (external temperature 100 ℃) oil bath. After completion of the reaction, to precipitate the crystals by the addition of ethanol and distilled water to the reaction solution. Thereafter, the crystals were filtered, washed with ethanol / water mixture 40mL, and naturally dried at room temperature for 12 hours and dried under reduced pressure at 40 ℃, KP-103 24.2g light yellow 87.3% (yield, HPLC purity 95.3 % I got).
1 H-NMR (500MHz, CDCl 3)
δ: 0.96 (3H, dd, J = 2.68, 7.08 Hz), 2.13-2.26 (4H, m), 2.35 (2H, br), 2.70 (2H, br) ,2.90-2 .94 (1H, q, J = 7.08 Hz), 4.64 (2H, s), 4.82 (1H, dd, J = 0.73, 14.39 Hz), 4.87 (1H, dd, J = 0.73, 14.39 Hz), 5.45 (1H, s), 6.72-6.81 (2H , m), 7.51 (1H, dt, J = 6.59, 9.03 Hz), 7.78 (1H, s),
8.02 (1H, s).
FAB-MS m / z: 349 [M + H] +
:86-89 ℃ melting point
Optical rotation: [α] D 25 -87 ~ -91 ° (C = 1.0, methanol)
“Drugs at FDA: JUBLIA”. Retrieved 26 June 2014.
| NDA Appl No | RLD | Active Ingredient | Dosage Form; Route | Strength | Proprietary Name | Applicant | |
|---|---|---|---|---|---|---|---|
| N203567 | Yes | EFINACONAZOLE | SOLUTION; TOPICAL | 10% | JUBLIA | DOW PHARM |
Patent Data
| Appl No | Prod No | US Patent No | Patent Expiration |
Drug Substance Claim |
Drug Product Claim |
Patent Use Code |
|
|---|---|---|---|---|---|---|---|
| N203567 | 001 | 7214506 | Oct 5, 2021 | U – 281 | |||
| N203567 | 001 | 8039494 | Jul 8, 2030 | U – 281 | |||
| N203567 | 001 | 8486978 | Oct 24, 2030 | Y |
Exclusivity Data
| Appl No | Prod No | Exclusivity Code | Exclusivity Expiration |
|---|---|---|---|
| N203567 | 001 | NCE | Jun 6, 2019 |
UPDATED
Efinaconazole
(2R,3R)-2-(2,4-Difluorophenyl)-3-(4-methylene-1-piperidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol
(2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidine-1-yl)-1-(1H-1,2,4-triazole-1-yl)-butane-2-ol
EFINACONAZOLE,
KP-103,
cas 164650-44-6, Efinaconazole [INN], UNII-J82SB7FXWB, AC1LAJ21, Efinaconazole [USAN:INN],
- Efinaconazole
- Jublia
- KP-103
- UNII-J82SB7FXWB
(2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylidenepiperidin-1-yl)-1-(1,2,4-triazol-1-yl)butan-2-ol
Molecular Formula: C18H22F2N4O Molecular Weight: 348.390286
 efinaconazole
efinaconazole
1H NMR PREDICT

………………………………………
13C NMR PREDICT
COSY PREDICT
HMBC PREDICT
...............................
ELABORATION
1H NMR PREDICT
13C NMR
PATENT
Example
1Production of
(2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol
(KP-103)21.26 g (119.4 mmol) of the 4-methylenepiperidine hydrobromide
(4-MP.HBr) obtained in Production 1 and 2.859 g (119.4 mmol) of lithium
hydroxide were added to 80 mL of acetonitrile and stirred for a while.
Thereafter, 20 g (79.6 mmol) of
(2R,3S)-2-(2,4-difluorophenyl)-3-methyl-2-[(1H-1,2,4-triazol-1-yl)methyl]oxirane
was added and the mixture was heated under reflux in an oil bath
(external temperature: 100° C.) for 14 hours. After the reaction
completed, ethanol and distilled water were added to the reaction
mixture, whereupon a crystal was precipitated. Thereafter, the crystal
was filtered off, washed with 40 mL of an ethanol/water mixture, dried
with air at room temperature and further dried under reduced pressure at
40° C. for 12 hours to give a pale yellow crystal of KP-103 in an
amount of 24.2 g (yield, 87.3%; purity on HPLC, 95.3%).
1H-NMR (500 MHz, CDCl3)δ: 0.96 (3H, dd, J=2.68, 7.08 Hz), 2.13-2.26 (4H, m), 2.35 (2H, br), 2.70 (2H, br), 2.90-2.94 (1H, q, J=7.08 Hz), 4.64 (2H, s), 4.82 (1H, dd, J=0.73, 14.39 Hz), 4.87 (1H, dd, J=0.73, 14.39 Hz), 5.45 (1H, s), 6.72-6.81 (2H, m), 7.51 (1H, dt, J=6.59, 9.03 Hz), 7.78 (1H, s), 8.02 (1H, s).
1H-NMR (500 MHz, CDCl3)δ: 0.96 (3H, dd, J=2.68, 7.08 Hz), 2.13-2.26 (4H, m), 2.35 (2H, br), 2.70 (2H, br), 2.90-2.94 (1H, q, J=7.08 Hz), 4.64 (2H, s), 4.82 (1H, dd, J=0.73, 14.39 Hz), 4.87 (1H, dd, J=0.73, 14.39 Hz), 5.45 (1H, s), 6.72-6.81 (2H, m), 7.51 (1H, dt, J=6.59, 9.03 Hz), 7.78 (1H, s), 8.02 (1H, s).
FAB-MS m/z: 349 [M+H]+
melting point: 86-89° C.
optical rotation: [α]D 25 −87 to −91° (C=1.0, methanol)
………………………………….
Journal of Organic Chemistry, 2014 , vol. 79, 7 pg. 3272 – 3278
A
new synthetic route, the shortest reported to date, to access a key
intermediate for the synthesis of various triazole antifungal agents was
developed. The elusive tetrasubstituted stereogenic center that is
essential in advanced triazole antifungal agents was constructed via the
catalytic asymmetric cyanosilylation of a ketone. The subsequent
transformations were performed in two one-pot operations, enhancing the
overall synthetic efficiency toward the intermediate. This streamlined
synthetic approach was successfully applied to efficient
enantioselective syntheses of efinaconazole (Jublia) and ravuconazole.
Scheme 3. Synthesis of Efinaconazole (Jublia)
(2R,3R)-2-(2,4-Difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol (Efinaconazole)
To a solution of 1 (54.2
mg, 0.216 mmol) in EtOH (217 μL) was added 4-methylenepiperidine (147
mg, 1.51 mmol), ……………………deleted………………. see original…………….. was purified
using silica gel column chromatography (CHCl3/MeOH = 10:1) to give 67.6 mg ofefinaconazole (90% yield) as a colorless amorphous solid.
[α]D20 −87.8 (c 1.12, CHCl3);
1H NMR (400 MHz, CDCl3) δ 8.00 (s, 1H), 7.76 (s, 1H), 7.51–7.45 (m, 1H), 6.78–6.68 (m, 2H), 5.50 (brs, 1H), 4.85 (d,J = 14.4 Hz, 1H), 4.78 (d, J = 14.4 Hz, 1H), 4.61 (s, 2H), 2.88 (q, J = 6.9 Hz, 1H), 2.66 (br s, 2H), 2.32 (br s, 2H), 2.21–2.17 (m, 4H), 0.93 (dd, J = 6.9, 2.1 Hz, 3H);
13C NMR (150 MHz, CDCl3) δ 162.5 (dd, J = 250, 13 Hz), 158.5 (dd, J = 246, 12 Hz), 151.3, 145.9, 144.4, 130.6 (dd, J = 8.7, 5.8 Hz), 124.7 (dd, J= 14, 3.6 Hz), 111.4 (dd, J = 20, 2.9 Hz), 108.1, 104.1 (dd, J = 28, 25 Hz), 77.7 (d, J = 5.8 Hz), 64.4, 55.9 (d, J = 8.7 Hz), 52.4, 35.2, 7.63 (d, J = 2.9 Hz);
19F NMR (376 MHz, CDCl3) δ −105.8, −110.7;
IR (CHCl3, cm–1) ν 3423, 3073, 2979, 2939, 2899, 2810, 1615, 1498, 1418, 1273, 1138;
HRMS (ESI-TOF) calcd for C18H23ON4F2 [M + H]+ m/z 349.1834, found 349.1828.
……………
SYN
http://newdrugapprovals.org/2014/06/10/valeant-pharmaceuticals-announces-fda-approval-of-jublia-for-the-treatment-of-onychomycosis/
updated
1H NMR
get clear pic at........http://pubs.acs.org/doi/suppl/10.1021/jo500369y/suppl_file/jo500369y_si_001.pdf
13C NMR
get clear pic at........http://pubs.acs.org/doi/suppl/10.1021/jo500369y/suppl_file/jo500369y_si_001.pdf
 amcrasto@gmail.com
amcrasto@gmail.com
 LIONEL MY SON
LIONEL MY SON
He was only in first standard in school when I was hit by a deadly one
in a million spine stroke called acute transverse mylitis, it made me
90% paralysed and bound to a wheel chair, Now I keep him as my source of
inspiration and helping millions, thanks to millions of my readers who
keep me going and help me to keep my son happy














This comment has been removed by the author.
ReplyDeleteAntifungal wholesale
ReplyDelete