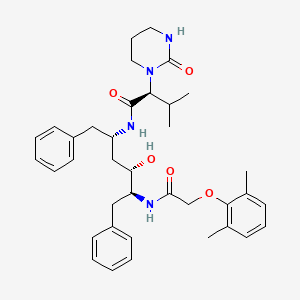
LOPINAVIR
(2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide
[1S-[1R*,(R*),3R*,4R*]]-N-[4-[[(2,6-dimethyl-phenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-alpha-(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetamide
(2S,3S,5S)-2-(-2,6- dimethylphenoxyacetyl)-amino-3-hydroxy-5-(2-(1-tetrahydropyrimid-2-onyl)-3- methylbutanoyl)amino-1 ,6-diphenylhexane
628.8008
| Abbott Laboratories |
| CAS | 192725-17-0 |
|---|
| AHFS/Drugs.com | International Drug Names |
|---|---|
| MedlinePlus | a602015 |
| Pregnancy cat. | C (US) |
| Legal status | POM (UK) ℞-only (US) |
US5914332
SYNONYMS
ABT-378, Aluviran, Koletra, ABT 378, 1mui, 2rkf, 2rkg, A 157378.0, RS-346
Molecular Formula: C37H48N4O5 Molecular Weight: 628.80082
Org. Proc. Res. Dev., 2000, 4 (4), pp 264–269
DOI: 10.1021/op990202j
http://pubs.acs.org/doi/abs/10.1021/op990202jA large scale process for the synthesis of HIV protease inhibitor candidate ABT-378 has been developed which utilizes an intermediate common to the synthesis of ritonavir, Abbott’s first generation compound. The synthesis relies on the sequential acylation of this intermediate which is carried through as a mixture of diastereomers until the penultimate step. A synthesis of acid 5, derived from l-valine, is also reported.
[1S-[1R*(R*),3R*,4R*]]-N-[4-[[(2,6-dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-α-(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetamide (2).
A 500-mL, three-necked, round-bottomed flask equipped
with mechanical stirring, ……………………..DELETED…………………The solid product was
washed with 30 mL of 1:1 EtOAc/heptane and dried in vacuo at 70 °C for
60 h, affording 18.8 g (89% yield) of ABT-378 2 as a colorless solid. Before crystallization crude 2 assayed as >93% pure by HPLC; after crystallization >99% purity was achieved.
mp (EtOAc), 124−127 °C. (uncorrected)
IR: 3413, 3335, 3289, 3060, 2966, 1671, 1650, 1624, 1545, 1520, 1453, 1189, 701 cm-1.
1H NMR (300 MHz): δ 7.30−7.13 (m, 10H), 7.02−6.92 (m, 3H), 6.86 (v br s, 1H), 5.68 (br s, 1H), 4.25 (m, 1H), 4.19 (app d, J = 10 Hz, 2H), 4.19 (m, 2H), 3.78 (m, app d sept, 1H), 3.12 (m, 1H), 3.06 (m, 2H), 2.97 (d, J = 7.6 Hz, 2H), 2.88 (m, 1H), 2.81 (app ABX dd, J = 14, 5.2 Hz, 1H), 2.68 (app ABX, dd, J = 14, 9.5 Hz, 1H), 2.23 (m, 1H), 2.18 (s, 6H), 1.83 (s, 1H), 1.74 (m, 2H), 1.53 (m, 1H), 1.28 (m, 2H), 0.83 (app t, J = 7 Hz, 6H).
13C NMR (75 MHz): δ 170.7, 168.8,
156.5, 154.2, 138.1, 138.0, 130.3, 129,3, 129.2, 129.0, 128.4, 128.2,
126.3, 126.0, 124.6, 70.2, 69.7, 63.1, 54.4, 48.7, 41.8, 41.1, 40.8,
40.0, 38.2, 25.4, 21.7, 19.6, 18.7, 16.1,
MS (ESI) 629 (M + H)+, 651 (M + Na)+.
Anal. Calcd for C37H48N4O5: C, 70.66; H, 7.69; N 8.91. Found: C, 70.26; H, 7.73; N 8.79.
[α]d20 = − 22.85 (c 0.4 MeOH).
-
Crystallographic studies have shown, to our surprise, that 2 isolated by this crystallization method is not a solvate.
-
The determination of the enantiomeric excess (% ee) for ABT-378 (2) can be done indirectly. Compound 17, which results from the acylation of 4 with the enantiomer of acid 5, is known to us, having been detected as an impurity in our process development.17 Compound 18 can only result from the acylation of the enantiomer of 4 (2R,3R,5R) with 5. The levels of 17/18 observed in 2 are typically <0.1%. Until there is a need for a more definitive assay, we assume this represents an upper limit to the amount of ent-2 present.
Enantiomeric excess
is determined by HPLC (Chiracel OD column, elution with hexane:
ethanol: trifluoroacetic acid (930: 70: 1). The desired l-isomer has a retention time of approximately 14 min; the d-isomer, 11.5 min.
References
- “FDA Approved Drug Products: Kaletra”. Retrieved 30 April 2004.
- KALETRA (lopinavir/ritonavir) capsules; (lopinavir/ritonavir) oral solution. Prescribing information. April 2009
- Capparelli E, Holland D, Okamoto C, et al. (2005). “Lopinavir concentrations in cerebrospinal fluid exceed the 50% inhibitory concentration for HIV”. AIDS (London, England) 19 (9).
- HIV drug used to reverse effects of virus that causes cervical cancer University of Manchester, 17 February 2014.
8-20-2003
|
Crystalline pharmaceutical
|
|
12-27-2002
|
Compositions and methods for enhancing the
bioavailability of pharmaceutical agents |
|
10-13-2000
|
PREGELATINIZED STARCH IN A CONTROLLED
RELEASE FORMULATION |
|
6-20-1997
|
RETROVIRAL PROTEASE INHIBITING COMPOUNDS
|
8-8-2012
|
PROCESS FOR THE PREPARATION OF SUBSTANTIALLY
PURE (2S,3S,5S)-5-AMINO-2-N,N-DIBENZYLAMINO-3- HYDROXY-1,6-DIPHENYLHEXANE |
|
11-12-2010
|
PRODRUGS OF HIV PROTEASE INHIBITORS
|
|
5-19-2010
|
Prodrugs of HIV protease inhibitors
|
|
5-7-2010
|
DIMETHYLPHENOXY MODULATORS OF VIRAL
PROTEASE ACTIVITY AND/OR PARASITIC ENZYME ACTIVITY |
|
1-12-2007
|
Methods of treating cancer
|
|
9-21-2005
|
Method to design therapeutically important compounds
|
|
6-10-2005
|
Crystalline pharmaceutical
|
|
3-9-2005
|
Crystalline pharmaceutical
|
|
2-4-2005
|
Methods and
compositions for the treatment or prevention
of human immunodeficiency virus and related conditions using cyclooxygenase-2 selective inhibitors and antiviral agents |
|
8-27-2004
|
Methods of treating cancer
|
No comments:
Post a Comment