
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC00497K, Communication
DOI: 10.1039/C6GC00497K, Communication
Xiaofeng Zhang, Sanjun Zhi, Wei Wang, Shuai Liu, Jerry P. Jasinski, Wei Zhang
A pot-economical synthesis involving two [3 + 2] cycloadditions for diastereoselective synthesis of novel triazolobenzodiazepine-containing polycyclic compounds
A pot-economical synthesis involving two [3 + 2] cycloadditions for diastereoselective synthesis of novel triazolobenzodiazepine-containing polycyclic compounds
A pot-economical and diastereoselective synthesis involving catalyst-free click reaction for fused-triazolobenzodiazepines
*Corresponding authors
aCentre for Green Chemistry and Department of Chemistry, University of Massachusetts Boston, 100 Morrissey Boulevard, Boston, USA
E-mail: wei2.zhang@umb.edu
E-mail: wei2.zhang@umb.edu
bJiangsu Key Laboratory for the Chemistry of Low-Dimensional Materials, Huaiyin Normal University, Huaian, PR China
cSchool of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an, PR China
dDepartment of Chemistry, Keene State College, Keene, USA
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC00497K
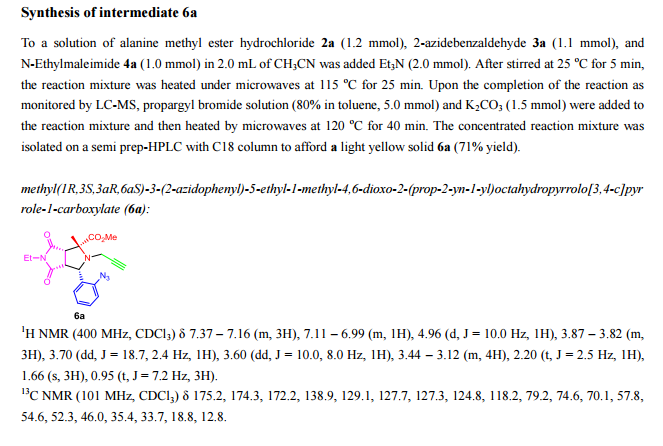
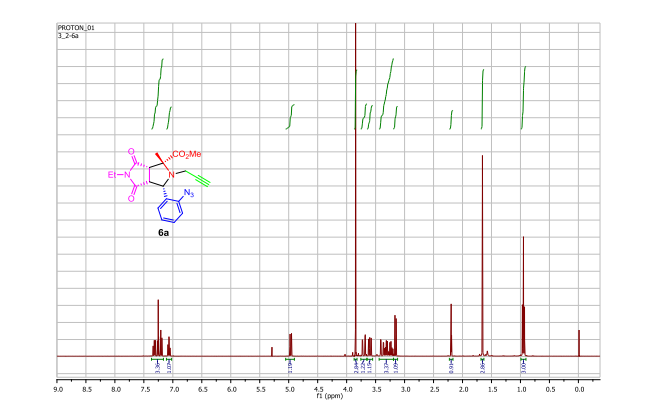
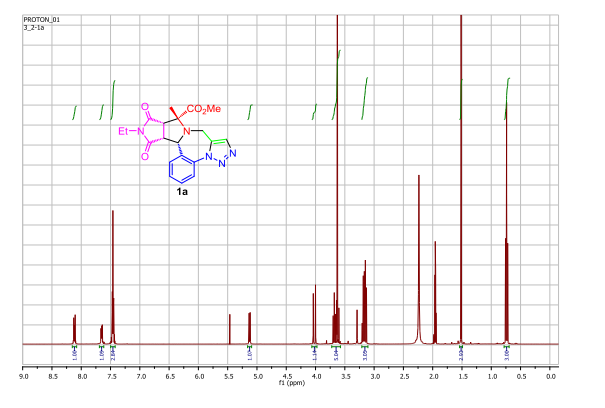
A pot-economical synthesis involving sequential [3 + 2] cycloadditions of an azomethine ylide and an azide–alkyne (click reaction) has been developed for diastereoselective synthesis of novel triazolobenzodiazepine-containing polycyclic compounds. A new example of catalyst-free click chemistry of non-strained alkynes is also disclosed
/////A pot-economical, diastereoselective synthesis, catalyst-free click reaction, fused-triazolobenzodiazepines

No comments:
Post a Comment