
We
present a series of oxadiazolothiazinones, selective inotropic agents
on isolated cardiac tissues, devoid of chronotropy and vasorelaxant
activity. Functional and binding data for the precursor of the series
(compound 1) let us hypothesize LTCC blocking activity and the
existence of a recognition site specific for this scaffold. We
synthesized and tested 22 new derivatives: introducing a para-methoxyphenyl at C-8 led to compound 12 (EC50 = 0.022 μM), twice as potent as its para-bromo analogue (1).
For 10 analogues, we extended the characterization of the biological
properties by including the assessment of metabolic stability in human
liver microsomes and cytochrome P450 inhibition potential. We observed
that the methoxy group led to active compounds with low metabolic
stability and high CYP inhibition, whereas the protective effect of
bromine resulted in enhanced metabolic stability and reduced CYP
inhibition. Thus, we identified two para-bromo benzothiazino-analogues as candidates for further studies.
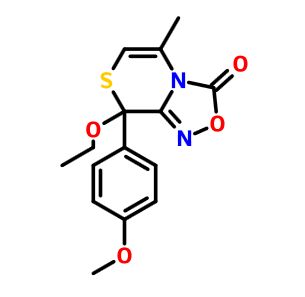
8-ethoxy-8-(4-methoxyphenyl)-5-methyl-8H-[1,2,4]oxadiazolo[3,4-c][1,4]thiazin-3-one
Understanding Oxadiazolothiazinone Biological Properties: Negative Inotropic Activity versus Cytochrome P450-Mediated Metabolism
† Dipartimento di Chimica, Biologia e Biotecnologie, Università di Perugia, Via Elce di Sotto 10, 06123 Perugia, Italy
‡ Centre for Drug Candidate Optimisation, Monash Institute of Pharmaceutical Sciences, 381 Royal Parade, Parkville, VIC 3052, Australia
§ Dipartimento di Farmacia, Università di Napoli “Federico II”, Via D. Montesano 49, 80131 Napoli, Italy
∥ Dipartimento di Farmacia e Biotecnologie, Università di Bologna, Via Belmeloro 6, 40126 Bologna, Italy
⊥ Dipartimento di Scienze della Vita, Università degli Studi di Siena, Via A. Moro 2, 53100 Siena, Italy
# Dipartimento di Neuroscienze, Area del Farmaco e Salute del Bambino (NEUROFARBA), Viale Pieraccini 6, 50139 Firenze, Italy
○ Dipartimento di Chimica ‘G. Ciamician’, Alma Mater Studiorum-Università di Bologna, Via Selmi 2, 40126 Bologna, Italy
J. Med. Chem., Article ASAP
DOI: 10.1021/acs.jmedchem.6b00030
Publication Date (Web): March 10, 2016
Copyright © 2016 American Chemical Society
/////////Cytochrome P450-Mediated Metabolism, Negative Inotropic Activity
CCOC2(c1ccc(OC)cc1)SC=C(C)n3c2noc3=O
Wilsons Promontory National Park, Korumburra, VIC, Australia
 .
.











///////
Wilsons Promontory National Park
National park in Victoria, Australia
The
Wilsons Promontory National Park, commonly known as Wilsons Prom or The
Prom, is a national park in the Gippsland region of Victoria,
Australia, located approximately 157 kilometres southeast of Melbourne. Wikipedia
 .
.


///////

No comments:
Post a Comment