| Molecular Formula: | C57H60N10O12S2 |
|---|---|
| Molecular Weight: | 1141.285 g/mol |
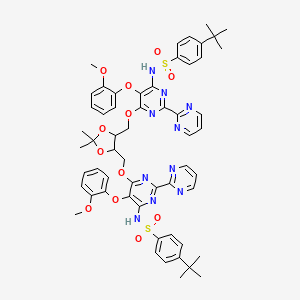
4-tert-butyl-N-[6-[[5-[[6-[(4-tert-butylphenyl)sulfonylamino]-5-(2-methoxyphenoxy)-2-pyrimidin-2-ylpyrimidin-4-yl]oxymethyl]-2,2-dimethyl-1,3-dioxolan-4-yl]methoxy]-5-(2-methoxyphenoxy)-2-pyrimidin-2-ylpyrimidin-4-yl]benzenesulfonamide
N,N′-(6,6′-(2,2-Dimethyl-1,3-dioxolane-4,5-diyl)bis-
(methylene)bis(oxy)bis(5-(2-methoxy phenoxy)-2,2′-bipyrimidine-6,4-diyl))bis(4-tert-butylbenzenesulfonamide)
Mp: 72−74 °C.
1
H NMR (400
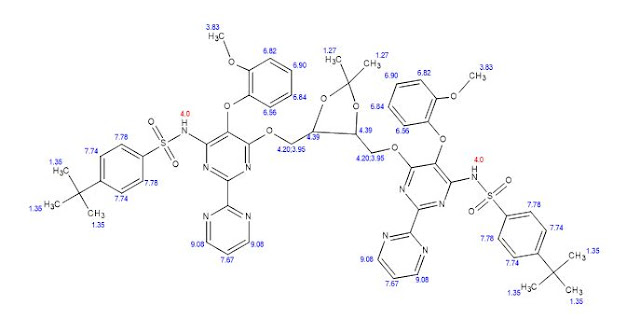
MHz, CDCl3): δ 1.25 (6H, s), 1.29 (18H, s), 3.84−3.90 (4H,
m), 4.27−4.31 (2H, m), 6.84−6.87 (3H, t), 6.97−7.00 (2H,
dd), 7.09−7.13 (3H, t), 7.43−7.45 (10H, m), 9.0−9.01 (4H,
d), 8.43 (2H, br s);
13C NMR (100 MHz, CDCl3): δ 25.88,
30.02, 34.10, 55.01, 61.53, 77.36, 108.43, 111.4, 118.73, 120.4,
124.09, 124.34, 126.67, 127.38, 128.35, 135.30, 138.25, 144.74,
148.62, 150.99, 156.07, 156.71, 160.56;
MS: m/z 1142.2 (M +
H);
Elem. Anal: Found: C 59.87, H 5.20, N 12.38; Calcd for
C57H60N10O12S2: C 59.99, H 5.30, N 12.27
A new and efficient synthetic process for the synthesis of an endothelin receptor antagonist, bosentan monohydrate, involves the coupling of p-tert-butyl-N-(6-chloro-5-(2-methoxy phenoxy)-2,2′-bipyrimidin-4-yl)benzenesulfonamide (7) with (2,2-dimethyl-1,3-dioxolane-4,5-diyl)dimethanol (14) as a key step. This new process provides desired bosentan monohydrate (1) with better quality and yields. Our new methodology consists of technical innovations/improvements which totally eliminate the probability for the formation of critical impurities such as pyrimidinone 8, dimer impurity 9, and N-alkylated impurity 13 in the final drug substance.
Org. Process Res. Dev., 2013, 17 (8), pp 1021–1026
DOI: 10.1021/op400100s
/////////////
CC1(OC(C(O1)COC2=NC(=NC(=C2OC3=CC=CC=C3OC)NS(=O)(=O)C4=CC=C(C=C4)C(C)(C)C)C5=NC=CC=N5)COC6=NC(=NC(=C6OC7=CC=CC=C7OC)NS(=O)(=O)C8=CC=C(C=C8)C(C)(C)C)C9=NC=CC=N9)C
CC1(OC(C(O1)COC2=NC(=NC(=C2OC3=CC=CC=C3OC)NS(=O)(=O)C4=CC=C(C=C4)C(C)(C)C)C5=NC=CC=N5)COC6=NC(=NC(=C6OC7=CC=CC=C7OC)NS(=O)(=O)C8=CC=C(C=C8)C(C)(C)C)C9=NC=CC=N9)C