One-step asymmetric synthesis of (R)- and (S)-rasagiline by reductive amination applying imine reductases
Green Chem., 2017, Advance Article
DOI: 10.1039/C6GC03023H, Communication
DOI: 10.1039/C6GC03023H, Communication
P. Matzel, M. Gand, M. Hohne
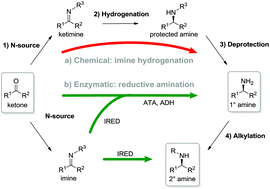
Imine reductases (IREDs) show great potential as catalysts for reductive amination of ketones to produce chiral secondary amines.
Imine reductases (IREDs) show great potential as catalysts for reductive amination of ketones to produce chiral secondary amines.
One-step asymmetric synthesis of (R)- and (S)-rasagiline by reductive amination applying imine reductases
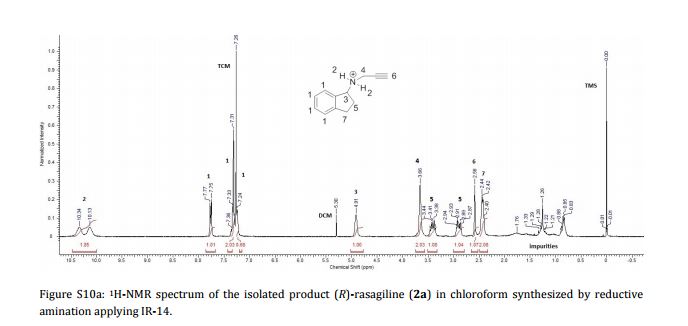
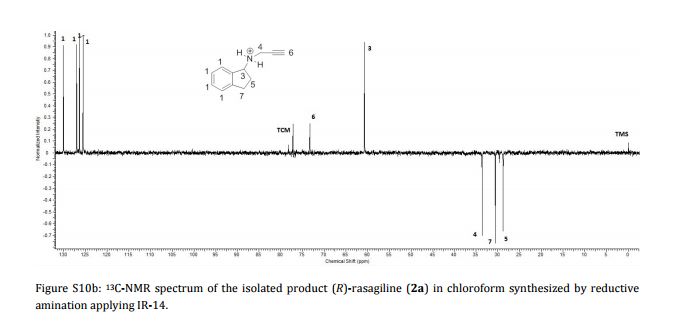
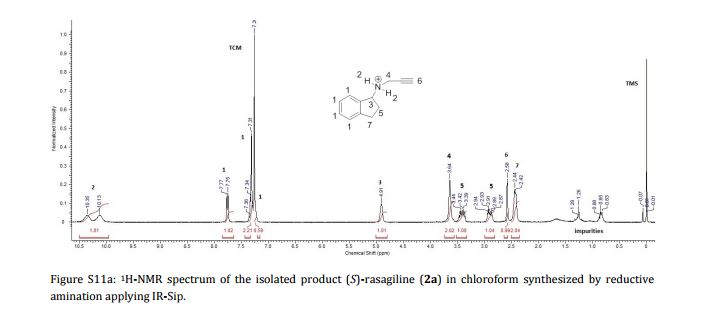
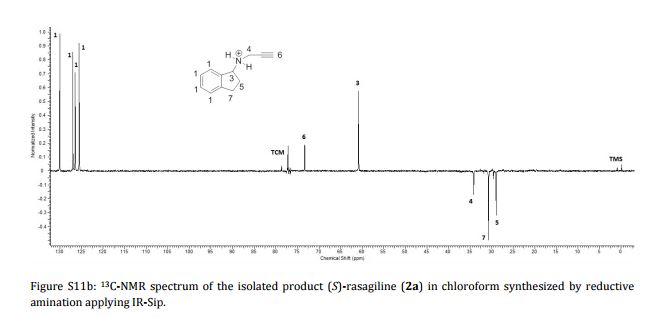
Imine reductases (IREDs) show great potential as catalysts for reductive amination of ketones to produce chiral secondary amines. In this work, we explored this potential and synthesized the pharmaceutically relevant (R)-rasagiline in high yields (up to 81%) and good enantiomeric excess (up to 90% ee) from the ketone precursor. This one-step approach in aqueous medium represents the shortest synthesis route from achiral starting materials. Furthermore, we demonstrate for the first time that tertiary amines also can be accessed by this route, which provides new opportunities for eco-friendly enzymatic asymmetric syntheses of these important molecules.
One-step asymmetric synthesis of (R)- and (S)-rasagiline by reductive amination applying imine reductases
*Corresponding authors
aInstitute of Biochemistry, Greifswald University, Felix-Hausdorff-Str. 4, 17487 Greifswald, Germany
E-mail: Matthias.Hoehne@uni-greifswald.de
E-mail: Matthias.Hoehne@uni-greifswald.de
bBiocenter Klein Flottbek, University of Hamburg, Ohnhorststr. 18, 22609 Hamburg, Germany
Green Chem., 2017, Advance Article
DOI: 10.1039/C6GC03023H
////////////One-step, asymmetric synthesis, (R)- , (S)-rasagiline, reductive amination, imine reductases
No comments:
Post a Comment