2-[4-(4-Chlorophenyl)piperazin-1-yl]-2-methylpropanoic Acid Ethyl Ester
1-Piperazineacetic
2-[4-(4-Chlorophény
Ethyl 2-[4-(4-chlor
Ethyl-2-[4-(4-chlor
1206769-44-9
2-[4-(4-Chloropheny
AGN-PC-0JIRMK
AKOS016034964
ethyl 2-[4-(4-chlor
MWt310.819
MFC16H23ClN2O2
1H NMR PREDICT
ACTUAL VALUES........1H NMR (400 MHz, CDCl3): δ ppm 1.27 (t, 3H, J = 7.2 Hz, -CH2-CH3), 1.35 (s, 6H, 2 x CH3), 2.74-2.76 (m, 4H, J = 4.8 Hz, -CH2-N-CH2-), 3.14-3.17 (m, 4H, J = 4.8 Hz, -CH2-N-CH2-), 4.20 (q, 2H, J = 7.2 Hz, -CH2-CH3), 6.81-6.83 (d, 2H, J = 6.8 Hz, phenyl protons), 7.17-7.20 (d, 2H, J = 6.8 Hz, phenyl protons).
13C NMR PREDICT
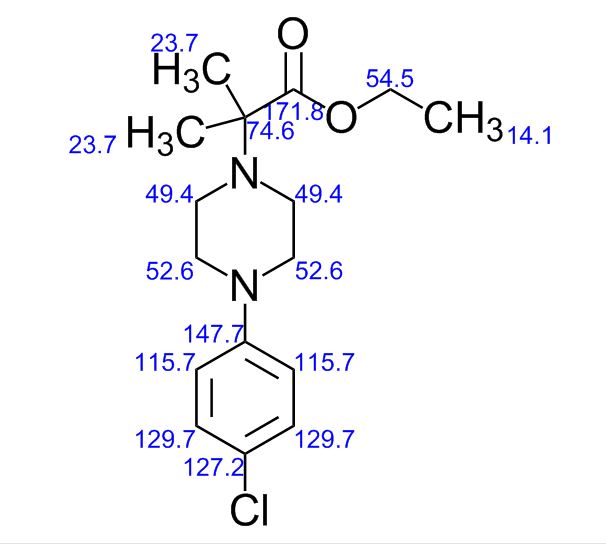
ACTUAL VALUES........13C NMR (100 MHz, CDCl3): δ ppm 14.3 (CH3), 22.7 ((CH3)2), 46.6 (-CH2-N-CH2-), 49.7 (-CH2-N-CH2-), 60.5 (O-CH2), 62.4 (N-C-), 117.0, 124.3, 128.8, 149.8 (aromatic carbons), 174.3 (C=O).
Paper
To a solution of 4-(4-chlorophenyl)piperazine dihydrochloride 1 (5.0 g, 0.0185 mol) in DMSO (30 ml), anhydrous cesium carbonate (30.0 g, 0.0925 mol), sodium iodide (1.39 g, 0.0093 mol) and ethyl 2-bromo-2-methylpropanoate 2 (3.97 g, 0.02 mol) were added. The resulting mixture was stirred at 25-30oC for 12 hours. The reaction mass was diluted with water (200 ml) and extracted with ethyl acetate (2 x 200 ml). The ethyl acetate layer was washed with water (2 x 100 ml), dried over anhydrous sodium sulfate (10.0 g) and concentrated under vacuum. The crude product thus obtained was purified by column chromatography (stationary phase silica gel 60-120 mesh; mobile phase 10% ethyl acetate in hexane). The title compound 3 was obtained as a white solid (4.73 g, 82 %).

Melting Point: 56oC.
EI-MS m/z (rel. int. %): 311 (100) [M+1]+, 236(40), 197(60), 154(45).
IR ν max (KBr) cm-1: 2839-2996 (C-H aliphatic); 1728 (C=O), 1595, 1505 (C=C aromatic), 1205 (C-O bending), 758 (C-Cl bending).
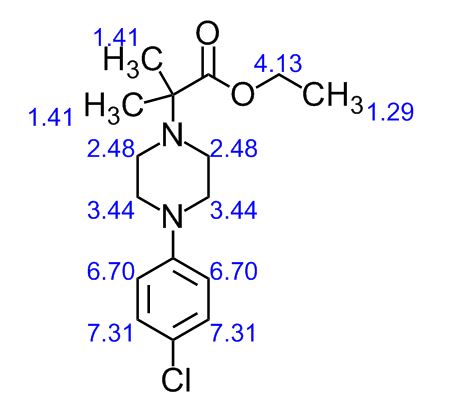
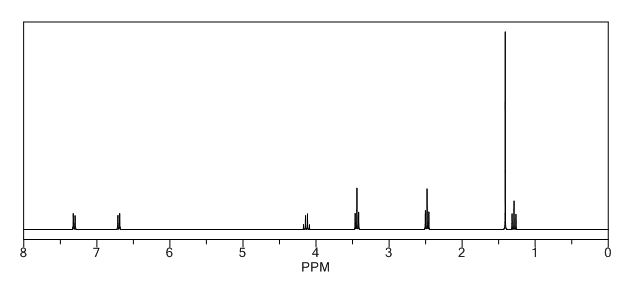
1H NMR (400 MHz, CDCl3): δ ppm 1.27 (t, 3H, J = 7.2 Hz, -CH2-CH3), 1.35 (s, 6H, 2 x CH3), 2.74-2.76 (m, 4H, J = 4.8 Hz, -CH2-N-CH2-), 3.14-3.17 (m, 4H, J = 4.8 Hz, -CH2-N-CH2-), 4.20 (q, 2H, J = 7.2 Hz, -CH2-CH3), 6.81-6.83 (d, 2H, J = 6.8 Hz, phenyl protons), 7.17-7.20 (d, 2H, J = 6.8 Hz, phenyl protons).
13C NMR (100 MHz, CDCl3): δ ppm 14.3 (CH3), 22.7 ((CH3)2), 46.6 (-CH2-N-CH2-), 49.7 (-CH2-N-CH2-), 60.5 (O-CH2), 62.4 (N-C-), 117.0, 124.3, 128.8, 149.8 (aromatic carbons), 174.3 (C=O).
Elemental analysis: Calculated for C16H23ClN2O2: C, 61.83%, H, 7.46%, N, 9.01%; Found: C, 61.90%, H, 7.44%, N, 8.98%.
Molbank 2009, 2009(3), M607; doi:10.3390/M607
Synthesis of 2-[4-(4-Chlorophenyl)piperazin-1-yl]-2-methylpropanoic Acid Ethyl Ester
1Department of Chemistry, Sambalpur University, JyotiVihar-768019, Orissa, India
2Institute of Chemical Technology (ICT), Matunga, Mumbai-400019, Maharashtra, India
*Author to whom correspondence should be addressed.
Received: 17 May 2009 / Accepted: 30 June 2009 / Published: 27 July 2009

Professor at Sambalpur University, Chemistry Department
Abstract
The title compound was synthesized by N-alkylation of 4-(4-chlorophenyl)piperazine with ethyl 2-bromo-2-methylpropanoate and its IR, 1H NMR, 13C NMR and Mass spectroscopic data are reported.
/////////
CCOC(=O)C(N1CCN(CC1)c1ccc(cc1)Cl)(C)C
No comments:
Post a Comment