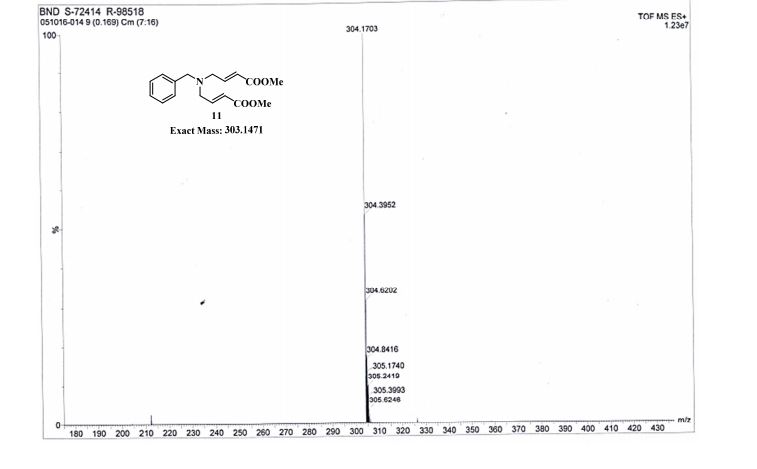
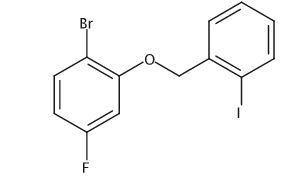
Dimethyl 4,4′-(Benzylazanediyl)(2E,2′E)-bis(but-2-enoate)
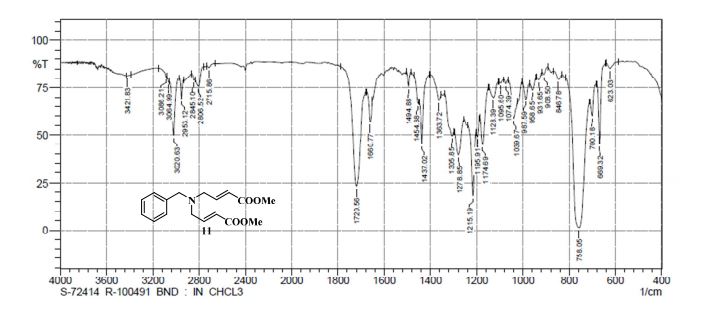
IR (CHCl3): ν = 758, 1215, 1278, 1437, 1660, 1720, 2806, 2953, 3020, 3421 cm–1;
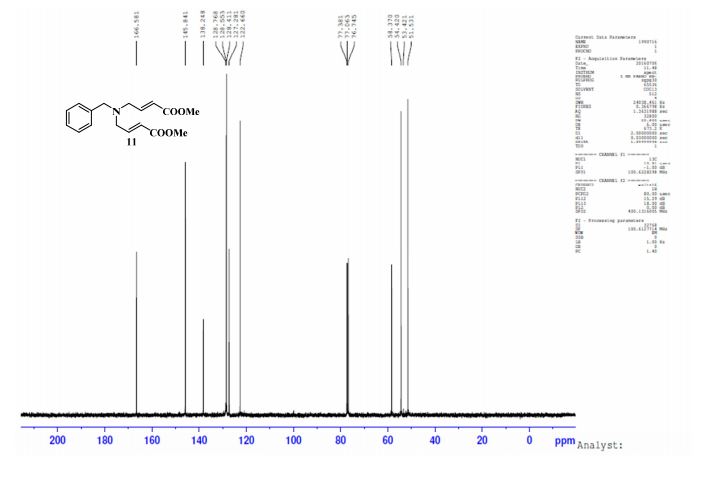
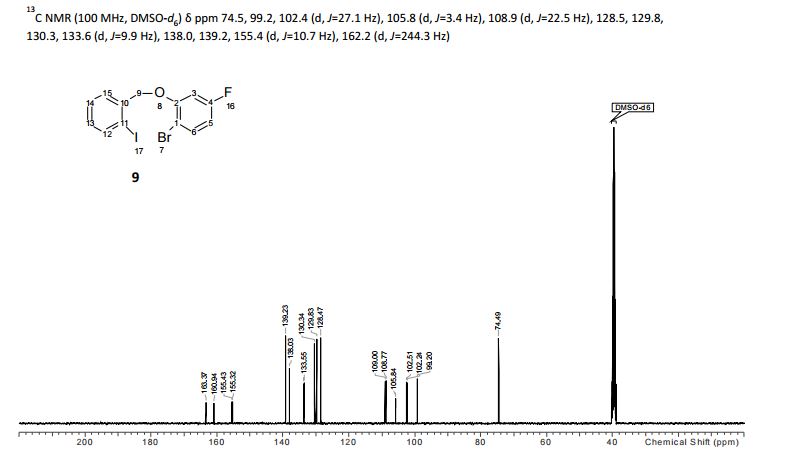
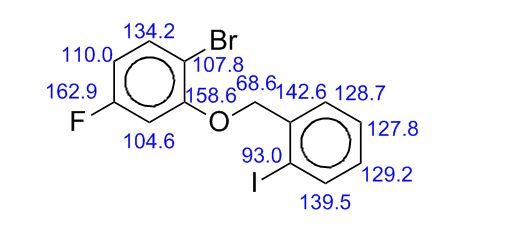
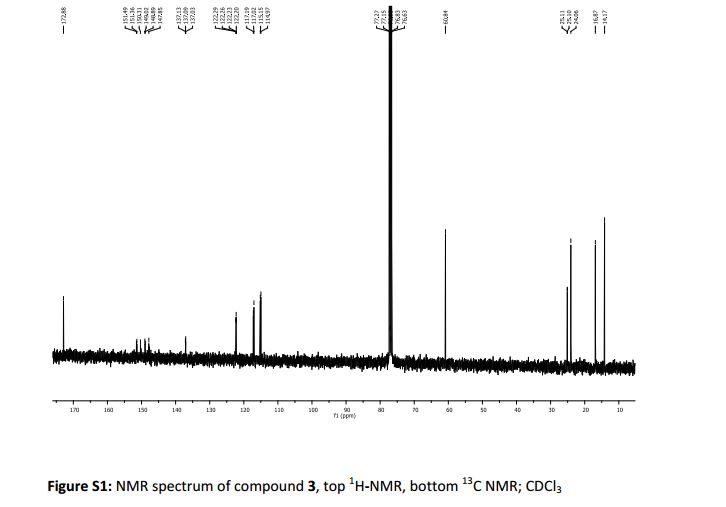
13C NMR (CDCl3, 100 MHz) δ: 51.53, 53.42, 58.37, 122.66, 127.28, 128.41, 128.55, 128.76, 138.24, 145.84, 166.58;
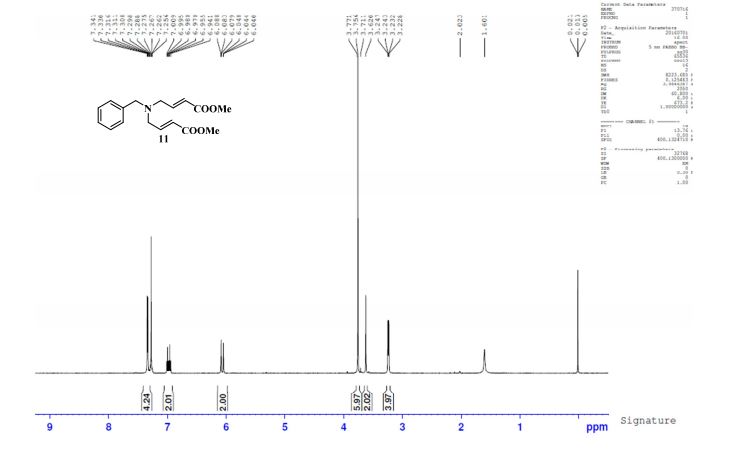
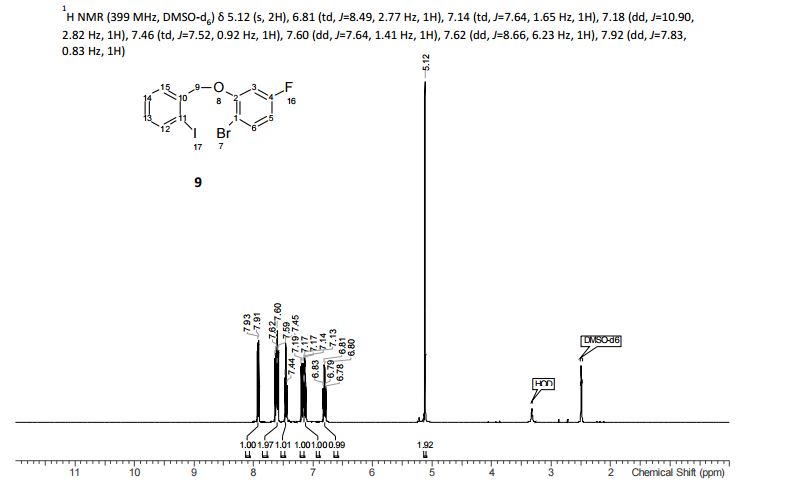
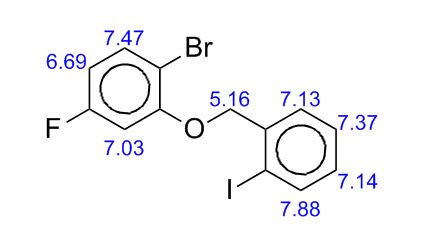
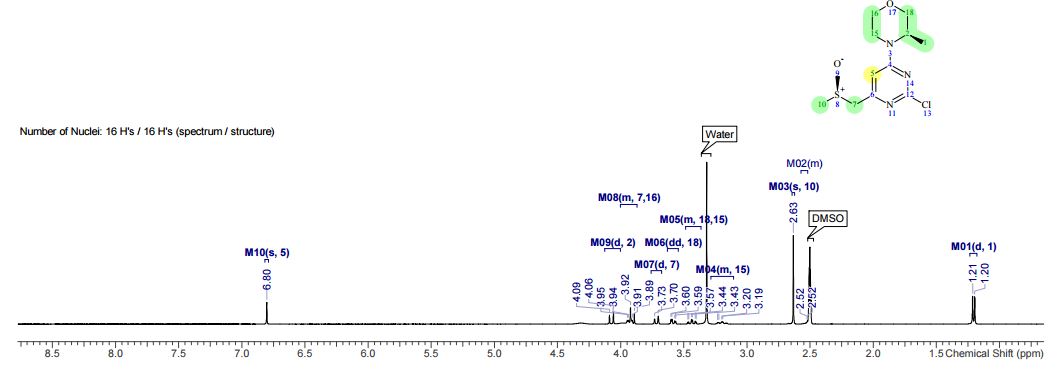
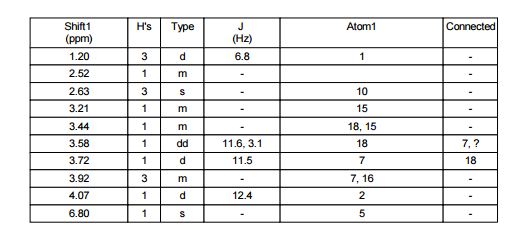
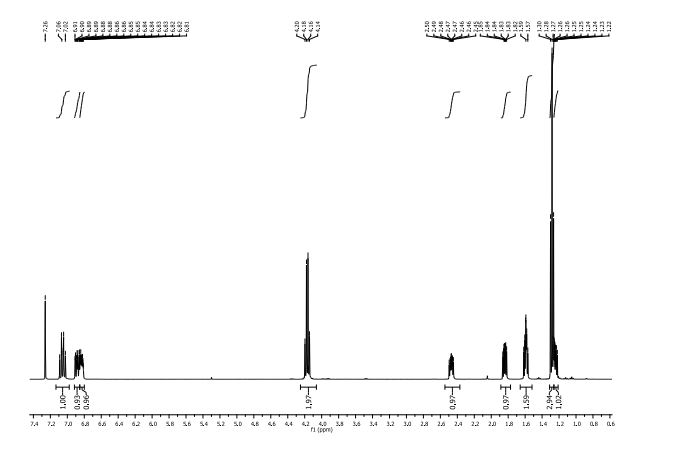
1H NMR (CDCl3, 400 MHz) δ: 3.23 (dd, J1 = 1.6 Hz, J2 = 6.0 Hz, 4H), 3.62 (s, 2H), 3.75 (s, 6H), 6.07 (dt, J1 = 1.6 Hz, J2 = 16.0 Hz, 2H), 6.97 (dt, J1 = 6.0 Hz, J2 = 16.0 Hz, 2H), 7.25–7.34 (m, 5H-merged with CDCl3 proton);
TOFMS: [C17H21NO4 + H+]: calculated 304.1543, found 304.1703(100%).
UPLC conditions were as follows for compound 11; Acquity Waters, column: BEH C18 (2.1 mm X 100 mm) 1.7 µm with mobile phases A (0.05% TFA in water) and B (acetonitrile). Detection was at 220 nm, flow was set at 0.4 mL/min, and the temperature was 30 °C (Run time: 9 min). Gradient: 0 min, A = 90%, B = 10%; 0.5 min, A = 90%, B = 10%; 6.0 min, A = 0%, B = 100%; 7.5 min, A = 0%, B = 100%; 7.6 min, A = 90%, B = 10%; 9.0 min, A = 90%, B = 10%.
Dimethyl 4,4′-(Benzylazanediyl)(2E,2′E)-bis(but-2-enoate) (11)
as a yellow oil. % purity: 93.4% (UPLC);
1H NMR (CDCl3, 400 MHz) δ: 3.23 (dd, J1 = 1.6 Hz, J2 = 6.0 Hz, 4H), 3.62 (s, 2H), 3.75 (s, 6H), 6.07 (dt, J1 = 1.6 Hz, J2 = 16.0 Hz, 2H), 6.97 (dt, J1 = 6.0 Hz, J2 = 16.0 Hz, 2H), 7.25–7.34 (m, 5H-merged with CDCl3 proton);
13C NMR (CDCl3, 100 MHz) δ: 51.53, 53.42, 58.37, 122.66, 127.28, 128.41, 128.55, 128.76, 138.24, 145.84, 166.58;
IR (CHCl3): ν = 758, 1215, 1278, 1437, 1660, 1720, 2806, 2953, 3020, 3421 cm–1;
TOFMS: [C17H21NO4 + H+]: calculated 304.1543, found 304.1703(100%).
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.6b00399
//////