
Methyl Nitroacetate (2)
Warning: Although no incidents occurred, the intermediates generated, as well as the end product, are energetic and should be handled as if they are explosive materials. It is essential that all reactions be conducted behind a blast shield and that proper protective equipment, including a face shield, be worn at all times during the operation.
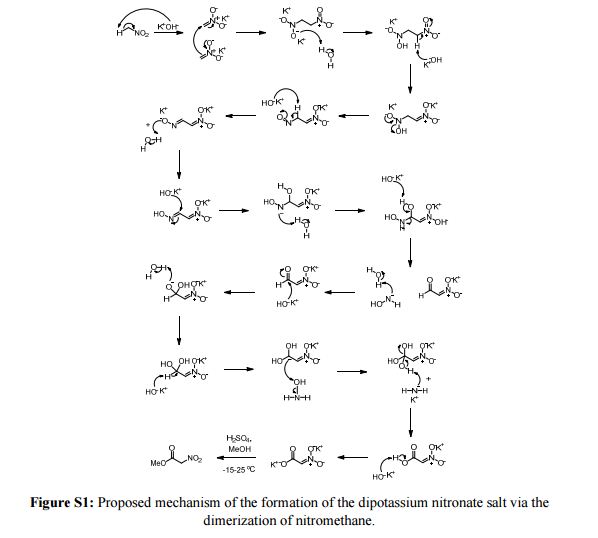
A new procedure for the synthesis and isolation of methyl nitroacetate is described. The previously published method required drying the explosive dipotassium salt of nitroacetic acid in a vacuum desiccator, followed by grinding this material into a fine powder with a mortar and pestle prior to esterification. To obtain the desired product, benzene was employed as the extraction solvent, sodium sulfate was used as the drying agent, and two distillations were required. The new procedure eliminates drying and grinding of the explosive dipotassium salt, employs ethyl acetate or dichloromethane as the extraction solvent, eliminates the need for a drying agent, and requires a single distillation to furnish the end product in high yield and purity.

clear colorless liquid, bp 65 °C (3.9 Torr).
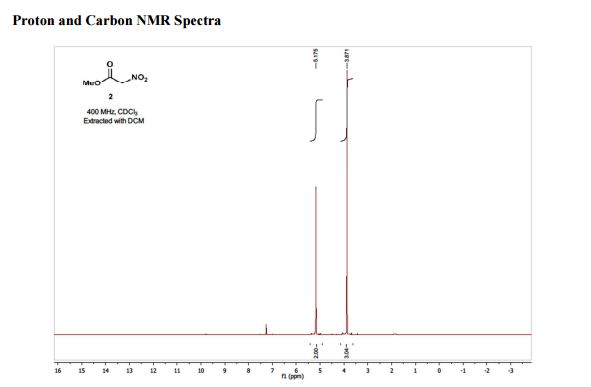
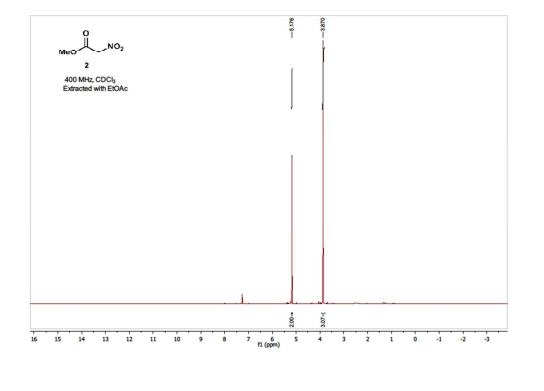
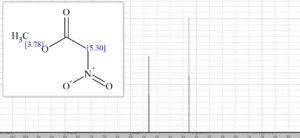
1H NMR (400 MHz; CDCl3) δ 5.18 (s, 2H), 3.87 (s, 3H);
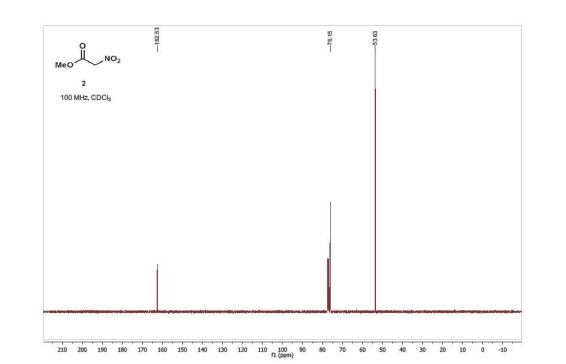
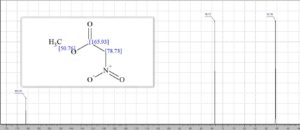
13C NMR (100 MHz, CDCl3) δ 162.5, 76.2, 53.6;
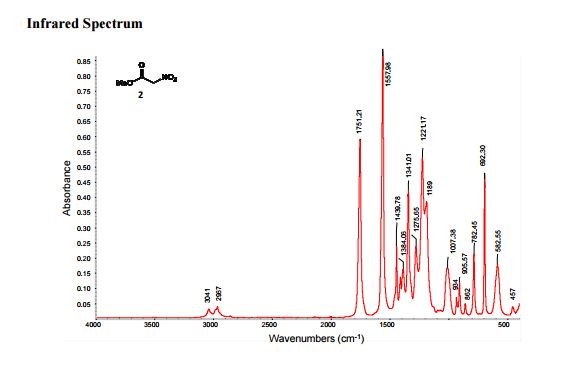
IR (neat): 3041, 2967, 1751, 1557;
Tdec= 251 °C (onset), 272 °C (peak).
NMR PREDICT


†Energetics Technology Branch, ‡Energetic Materials Science Branch, U.S. Army Research Laboratory, Aberdeen Proving Ground, Maryland 21005, United States
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00093
*E-mail: jesse.j.sabatini.civ@mail.mil. Phone: 410-278-0235., *E-mail: pablo.e.guzman2.civ@mail.mil. Phone: 410-278-8608.
NMR predict
//////////////////

This comment has been removed by the author.
ReplyDelete