Green Chem., 2017, Advance Article
DOI: 10.1039/C7GC01519D, Communication
DOI: 10.1039/C7GC01519D, Communication
D. J. M. Lyons, R. D. Crocker, D. Enders, T. V. Nguyen
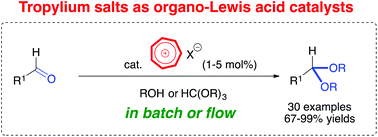
Tropylium salts were reported as organic-Lewis acids to efficiently catalyze acetalization reactions in batch and flow.
Tropylium salts were reported as organic-Lewis acids to efficiently catalyze acetalization reactions in batch and flow.
- From the journal:
Green Chemistry
Tropylium salts as efficient organic Lewis acid catalysts for acetalization and transacetalization reactions in batch and flow
Abstract
Acetalization reactions play significant roles in the synthetically important masking chemistry of carbonyl compounds. Herein we demonstrate for the first time that tropylium salts can act as organic Lewis acid catalysts to facilitate acetalization and transacetalization reactions of a wide range of aldehyde substrates. This metal-free method works efficiently in both batch and flow conditions, prompting further future applications of tropylium organocatalysts in green synthesis.
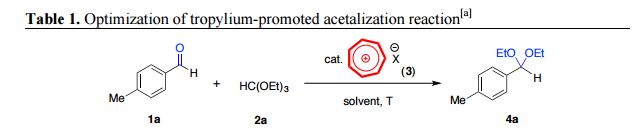
1-(Diethoxymethyl)-4-methylbenzene (4a):
Prepared according to the general procedure from p-tolualdehyde and triethylorthoformate to yield the title compound as a colourless oil (99 mg, 0.50 mmol, quant. yield). 4a
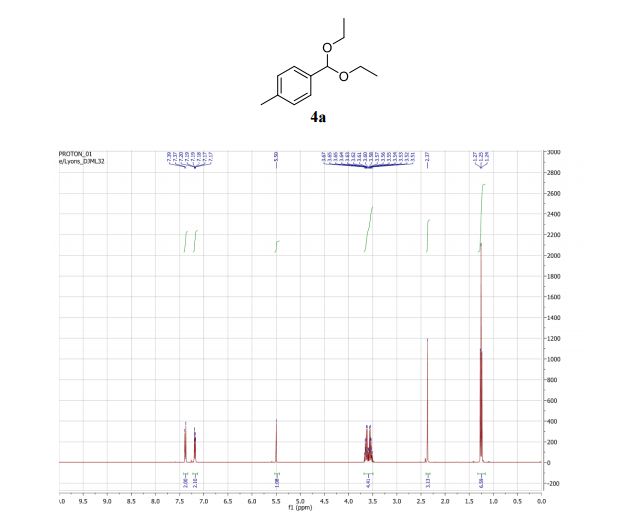
1 H NMR (400 MHz, CDCl3) δ 7.38 (d, J = 8.1 Hz, 2H), 7.18 (d, J = 7.9 Hz, 2H), 5.50 (s, 1H), 3.59 (ddq, J = 35.4, 9.5, 7.1 Hz, 4H), 2.37 (s, 3H), 1.25 (t, J = 7.1 Hz, 6H) ppm;
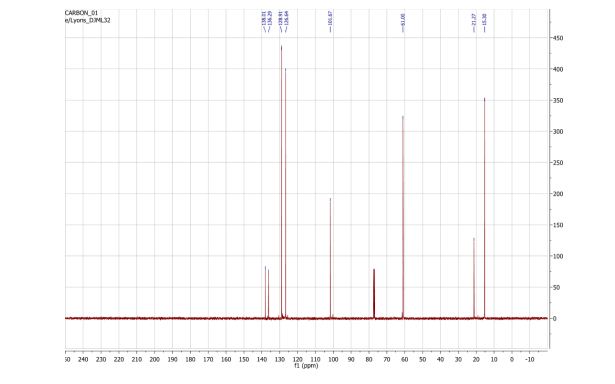
13C NMR (101 MHz, CDCl3) δ 138.0, 136.3, 128.9, 126.64, 101.7, 61.0, 21.3, 15.3 ppm;
IR (KBr) 2973, 2880, 2327, 2102, 1909, 1740, 1448 cm-1 ;
ESI-MS Anal. Calcd. for 217.1199 C12H18O2Na, found 217.1195
1H NMR
1 H NMR (400 MHz, CDCl3) δ 7.38 (d, J = 8.1 Hz, 2H), 7.18 (d, J = 7.9 Hz, 2H), 5.50 (s, 1H), 3.59 (ddq, J = 35.4, 9.5, 7.1 Hz, 4H), 2.37 (s, 3H), 1.25 (t, J = 7.1 Hz, 6H) ppm;
13c nmr

13C NMR (101 MHz, CDCl3) δ 138.0, 136.3, 128.9, 126.64, 101.7, 61.0, 21.3, 15.3 ppm;

Vinh Nguyen

BE (Hon 1, UNSW), Ph.D (ANU), MRACI CChem
Lecturer and DECRA fellow
Contact details
Phone: +61-2-9385 6167
Email: t.v.nguyen@unsw.edu.au
Email: t.v.nguyen@unsw.edu.au
Office
Room 217 Dalton Building (F12)
School of Chemistry - UNSW
Research Group Website
Biographical Details
Dr. Vinh Nguyen (also known as: Thanh Vinh Nguyen or Thanh V. Nguyen on academic publication) was born in Vietnam. After high school, he went to Sydney, Australia to study industrial chemistry at University of New South Wales. He then moved to undertake his PhD in organic chemistry with Professor Michael Sherburn at the Australian National University, Canberra. He had worked to develop new synthetic methodologies for application in natural product synthesis and worked on the design and synthesis of enormoussynthetic host molecules for drug-delivery modelling. After graduating in 2010, he came to work on organocatalysis in Professor Dieter Enders group at the Institute of Organic Chemistry, RWTH Aachen, Germany under the auspices of an Alexander von Humboldt Postdoctoral Fellowship. In June 2013, he moved to Curtin University (Perth, Australia) to start his own independent research group. In June 2015, he moved again to UNSW (Sydney) to take up a Lecturer/DECRA fellow position at the School of Chemistry. His current research interests are organocatalysis, aromatic cation activation, synthesis of naturally occurring and bioactive compounds, asymmetric synthesis and medicinal chemistry.
Selected Awards and Academic Achievements
- 2016: The 2016 Athel Beckwith Lectureship from RACI Organic Chemistry Division
- 2015-2018: ARC Discovery Early Career Researcher Award (DECRA)
- 2014: Thieme Chemistry Journal Award for outstanding early career academics.
- 2011 – 2013: Alexander von Humboldt Postdoctoral Fellowship (RWTH Aachen, Germany).
- 2005: The Era Polymer Prize for “The Best Honor Research Thesis” in Industrial Chemistry – UNSW
- 2000: Silver medal in the 32nd International Chemistry Olympiad in Copenhagen, Denmark
Research interests
Nguyen’s group focuses their research on development of new synthetic methodologies in organic chemistry, organocatalysis and natural product synthesis.
Aromatic Cation Activation:
A new method for the nucleophilic substitution of alcohols and carboxylic acids and other substrates using aromatic tropylium cation activation has been developed. It demonstrates, for the first time, the synthetic potential of tropylium cations in promoting chemical transformations. (http://pubs.acs.org/doi/abs/10.1021/ol5003972).

Fine writing ! Thank you so much for sharing this beautiful blog.
ReplyDeleteSell KU Research Chemical in USA,
Research Chemical KU Crystal Vendor in USA