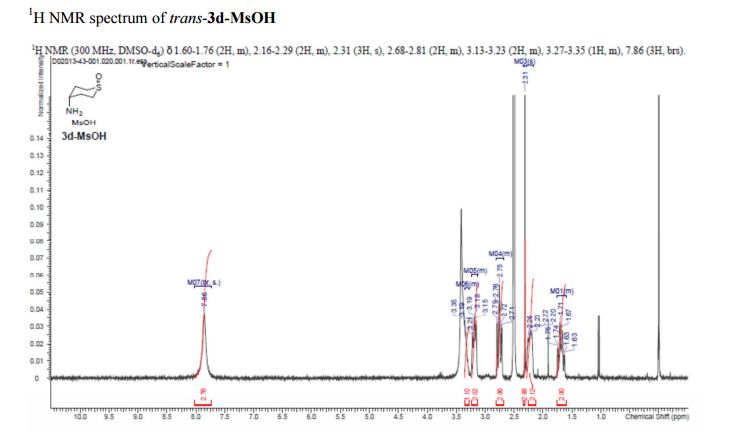

trans-4-Aminotetrahydro-2H-thiopyran 1-Oxide Methanesulfonate (trans-3d-MsOH)
Compound ...................afforded trans-3d-MsOH (20 g, 68%) as a white crystalline solid.
1H NMR (300 MHz, DMSO-d6) δ 1.60–1.76 (2H, m), 2.16–2.29 (2H, m), 2.31 (3H, s), 2.68–2.81 (2H, m), 3.13–3.23 (2H, m), 3.27–3.35 (1H, m), 7.86 (3H, brs).
13C NMR (75 MHz, D2O) δ 24.98, 38.51, 46.18, 46.84.
LCMS m/z calcd for C5H11NOS: 133.06, found 134.1 [M + H].
Anal. Calcd for C6H15NO4S2: C, 31.43; H, 6.59; N, 6.11. Found: C, 31.62; H, 6.48; N, 6.19. mp 214–216 °C.
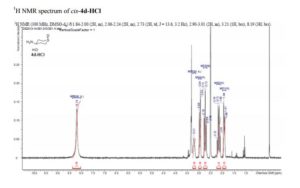
cis-4-Aminotetrahydro-2H-thiopyran 1-Oxide Hydrochloride (cis-4d-HCl)
A mixture of .................... to afford cis-4d-HCl (22.5 g, 62%) as a white crystalline solid.
1H NMR (400 MHz, DMSO-d6) δ 1.81–2.00 (2H, m), 2.06–2.24 (2H, m), 2.73 (2H, td, J = 13.6, 3.2 Hz), 2.90–3.01 (2H, m), 3.21 (1H, brs), 8.19 (3H, brs).
13C NMR (75 MHz, D2O) δ 19.80, 42.94, 47.34.
LCMS m/z calcd for C5H11NOS: 133.06, found 134.1 [M + H]. Anal. Calcd for C5H12NClOS: C, 35.39; H, 7.13; N, 8.26. Found: C, 35.28; H, 6.87; N, 8.26. mp 230–232 °C.
Efficient and Stereoselective Syntheses of Isomerically Pure 4-Aminotetrahydro-2H-thiopyran 1-Oxide Derivatives
† Research, Takeda Pharmaceutical Company Ltd., Fujisawa, Kanagawa 251-8555, Japan
‡ Pharmaceutical Sciences, Takeda Pharmaceutical Company Ltd., Yodogawa-ku, Osaka 532-8686, Japan
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00147
*E-mail: ryo.mizojiri@takeda.com. Phone: +81-466-32-1058 (R.M.)., *E-mail: tetsuji.kawamoto@takeda.com. Phone: +81-466-32-1193 (T.K.).

Efficient and stereoselective syntheses of isomerically pure 4-aminotetrahydro-2H-thiopyran 1-oxide derivatives have successfully been achieved. Isomerically pure (4-nitrophenyl)sulfonyltetrahydro-2H-thiopyran 1-oxides were identified by X-ray crystallographic analyses, and isomerically pure sulfoxide derivatives were characterized by 1H NMR. An oxidation reaction of tert-butyl(4-nitrophenyl)sulfonyl(tetrahydro-2H-thiopyran-4-yl)carbamate with Oxone provided steric control, affording its trans sulfoxide with high efficiency and selectivity. From the obtained trans sulfoxide derivatives, cis sulfoxide derivatives were synthesized conveniently by a hydrogen chloride catalyzed isomerization.


This comment has been removed by the author.
ReplyDelete