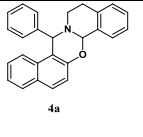
2H-1,3-Benzoxazine natural products and related bioactive molecules.
4-(2-Bromo-5-chlorobenzyl)-7-chloro-2-phenyl-2H-benzo[e][1,3]oxazine 2 as a light-yellow solid (82% yield).
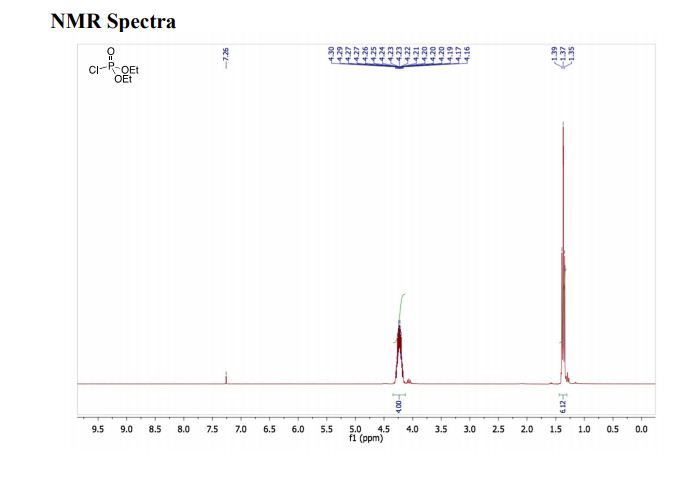
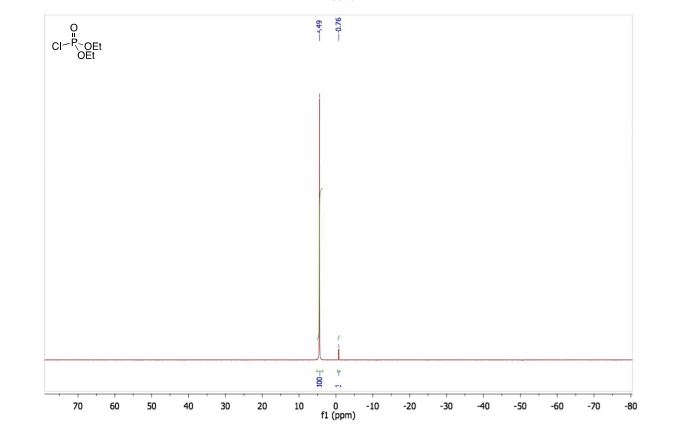
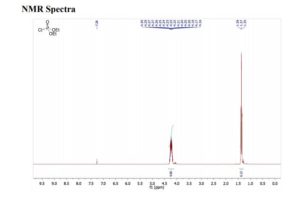
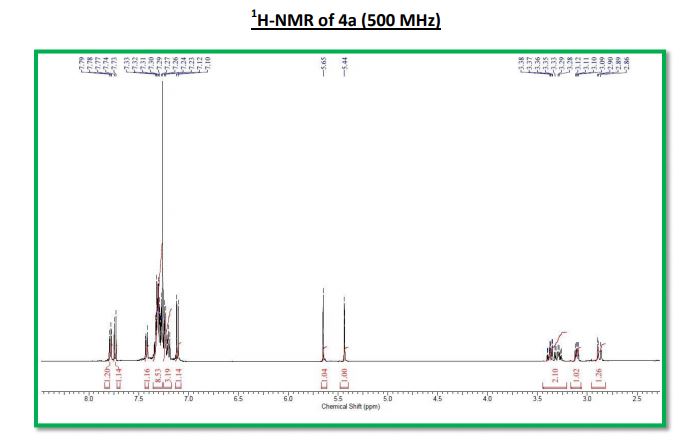
1H NMR (500 MHz, CDCl3): δ (ppm) 7.55–7.52 (m, 3H), 7.42–7.34 (m, 3H), 7.30 (d, 1H, J = 3.5 Hz,) 7.29 (s, 1H), 7.12 (dd, 1H, J = 8.5 Hz, 2.5 Hz), 6.95–6.91 (m, 2H), 6.57 (1H, s), 4.16 (ABq, 2H, ΔδAB = 0.05, JAB = 16.5 Hz).
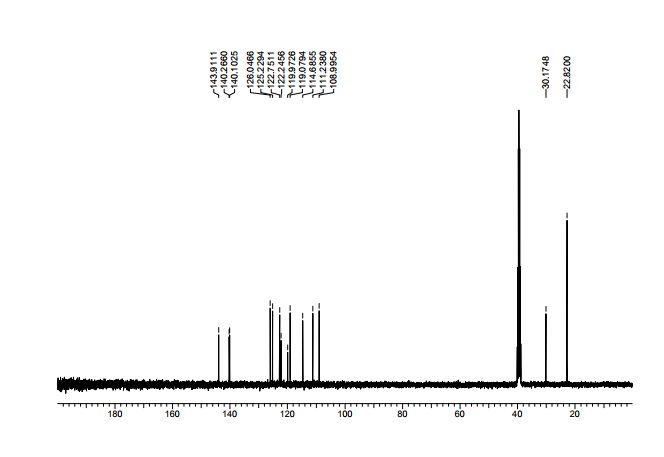
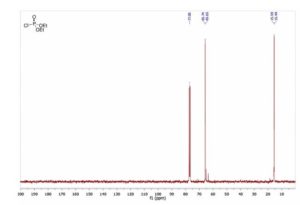
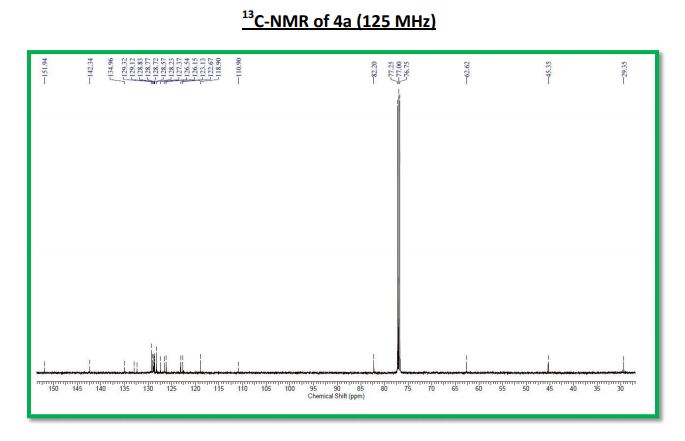
13C NMR (125 MHz, CDCl3): δ (ppm) 161.5, 155.8, 139.2, 138.9, 138.1, 133.8, 133.5, 130.6, 128.8, 128.7, 128.5, 127.0, 126.3, 122.6, 121.9, 117.3, 116.2, 88.9, 40.8.
HRMS TOF MS (m/z): [M + H]+ calcd for [C21H14BrCl2NO H] 445.9709; found 445.9713.
FTIR(neat): 3060, 1633, 1596, 1454, 1364, 1344 cm–1.
Spectroscopic data for 2 were identical to those reported in the literature.(4)
Li, H.; Belyk, K. M.; Yin, J.; Chen, Q.; Hyde, A.; Ji, Y.; Oliver, S.; Tudge, M.; Campeau, L.-C.; Campos, K. R. J. Am. Chem. Soc. 2015, 137,13728– 13731 DOI: 10.1021/jacs.5b05934
Development of a General Protocol To Prepare 2H-1,3-Benzoxazine Derivatives
† Department of Process Research and Development, MSD R&D (China) Co., Ltd., Building 21 Rongda Road, Wangjing R&D Base, Zhongguancun Electronic Zone West Zone, Beijing 100012, China
‡ Department of Process Research and Development, Merck Sharp & Dohme, Hertford Road, Hoddesdon, Hertfordshire EN11 9BU, United Kingdom
§ Department of Synthetic Chemistry, Pharmaron Beijing Co., Ltd., 6 Taihe Road BDA, Beijing, 100176, China
∥ Department of Process Research and Development, Merck Research Laboratories, P.O. Box 2000, Rahway, New Jersey 07065, United States
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00209
Publication Date (Web): August 23, 2017
Copyright © 2017 American Chemical Society
*E-mail: ji_qi@merck.com.
ACS Editors’ Choice – This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes.
Abstract
A practical synthesis and detailed development process of 2H-1,3-benzoxazine derivatives catalyzed by aldimine and trifluoromethanesulfonic acid is described. A broad range of substrates with diverse steric and electronic properties were explored. Aliphatic/aromatic/heteroaromatic substrates all proceed well under conditions which have been optimized into a robust, scalable process.
//////////