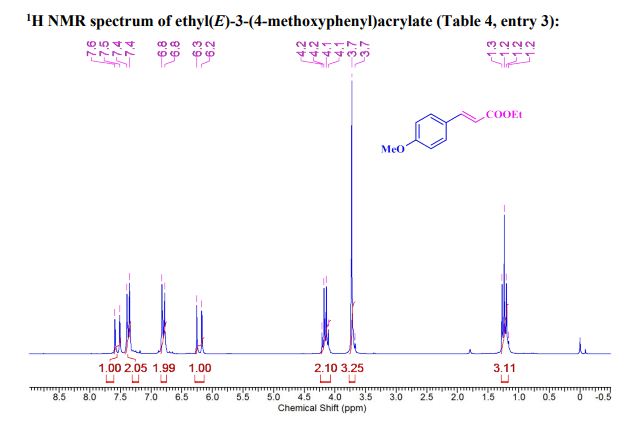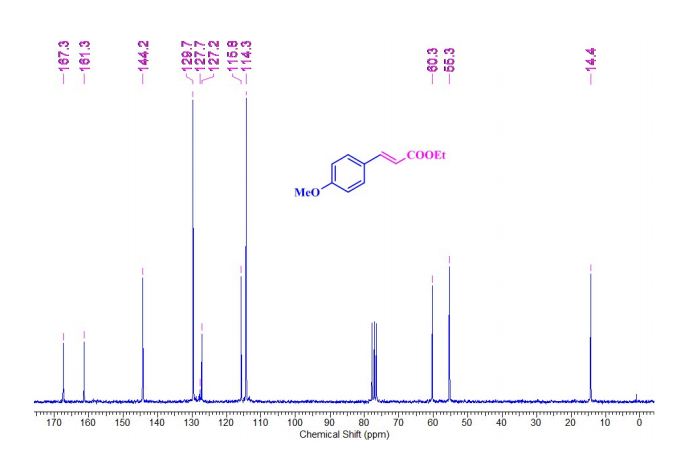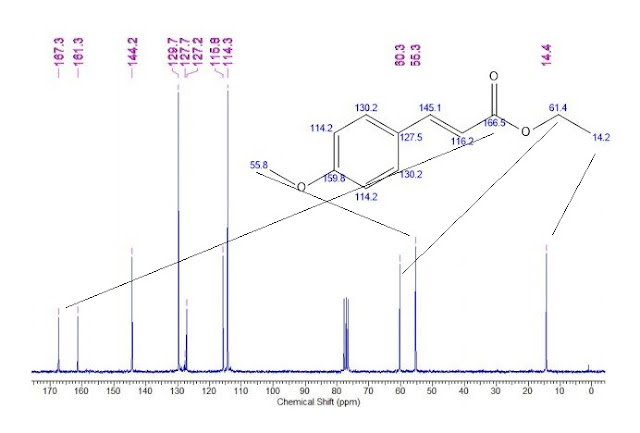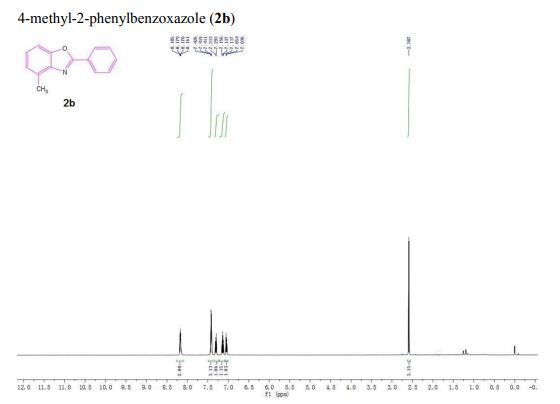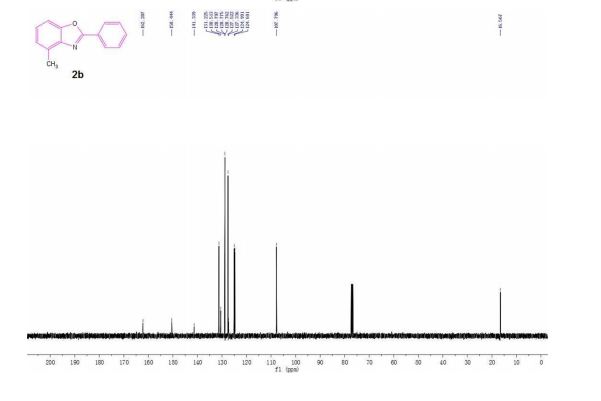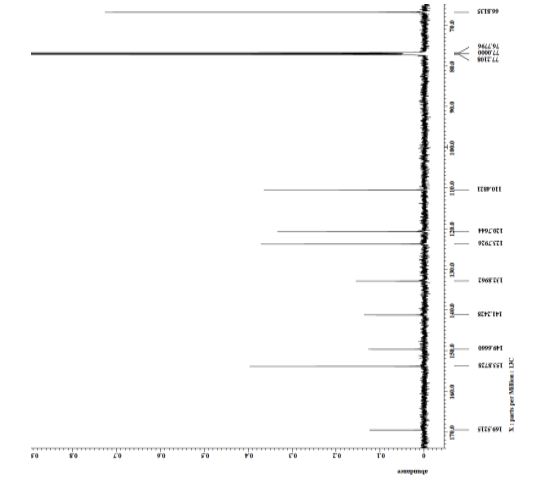

| 1465003-25-1 cas |
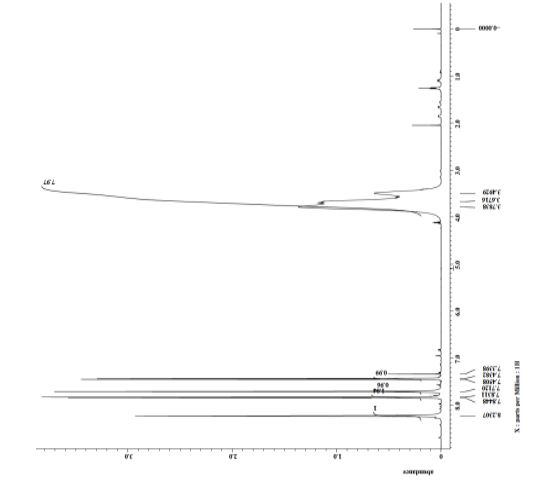
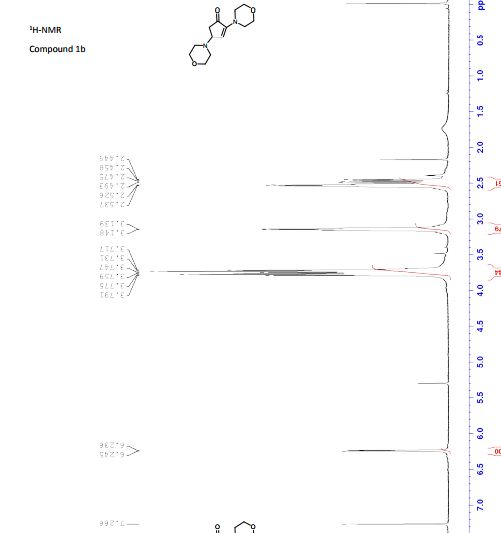
2,4-dimorpholinocyclopent-2-enone (1b):
1H NMR (300 MHz, CDCl3) 6.24 (d, J = 2.9 Hz, 1H, COC=CH), 3.78 (t, J = 4.7 Hz, 4H, morpholine), 3.73 (t, J = 4.7 Hz, 4H, morpholine), 3.73-3.72 (m, 1H, COCH2CHN), 3.15-3.14 (m, 4H, morpholine), 2.54-2.52 (m, 4H, morpholine), 2.49-2.48 (m, 1H, COCH2), 2.46-2.45 (m, 1H, COCH2);
13C NMR (75 MHz, CDCl3) 38.1, 48.1, 50.0, 60.3, 66.6, 67.1, 129.5, 151.7, 202.0.


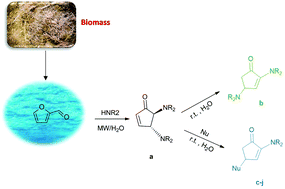
Water excellent solvent for the synthesis of bifunctionalized cyclopentenones from furfural
Abstract
Chiral cyclopentenones are important precursors in the asymmetric synthesis of target molecules. In particular, C-2 amino cyclopentenones could be utilised as intermediates towards antitumor natural products. On the basis of our previous experience, we report an environmentally friendly protocol for the synthesis of bifunctionalized cyclopentenones in water from furfural. The use of water and MW gives high regioselectivity and good to excellent yields. The reaction can be realized in short times with various nucleophiles.

//////////////

1 Estevão, Mónica S.; Afonso, Carlos A.M.; Tetrahedron Letters; vol. 58; nb. 4; (2017); p. 302 - 304
2 Green Chemistry; vol. 19; nb. 1; (2017); p. 164 - 168
“ORG SPECT INT” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent

“ORG SPECT INT” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent