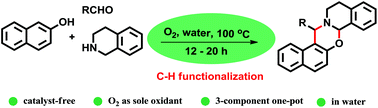
Catalyst-free multi-component cascade C-H-functionalization in water using molecular oxygen: an approach to 1,3-oxazines
Green Chem., 2017, 19,4036-4042
DOI: 10.1039/C7GC01494E, Communication
DOI: 10.1039/C7GC01494E, Communication
Mohit L. Deb, Choitanya D. Pegu, Paran J. Borpatra, Prakash J. Saikia, Pranjal K. Baruah
Synthesis of 1,3-oxazines via catalyst free C-H functionalization using molecular oxygen in water.
Synthesis of 1,3-oxazines via catalyst free C-H functionalization using molecular oxygen in water.
Catalyst-free multi-component cascade C–H-functionalization in water using molecular oxygen: an approach to 1,3-oxazines
Abstract
Herein, catalyst-free 3-component reactions of naphthols, aldehydes, and tetrahydroisoquinolines to synthesize 1,3-oxazines is reported. The reaction is performed in H2O in the presence of O2 as the sole oxidant at 100 °C, which proceeds through the formation of 1-aminoalkyl-2-naphthols followed by selective α-C–H functionalization of tert-amine.
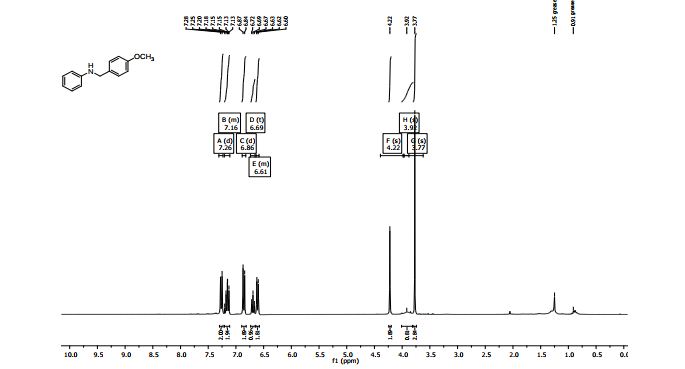
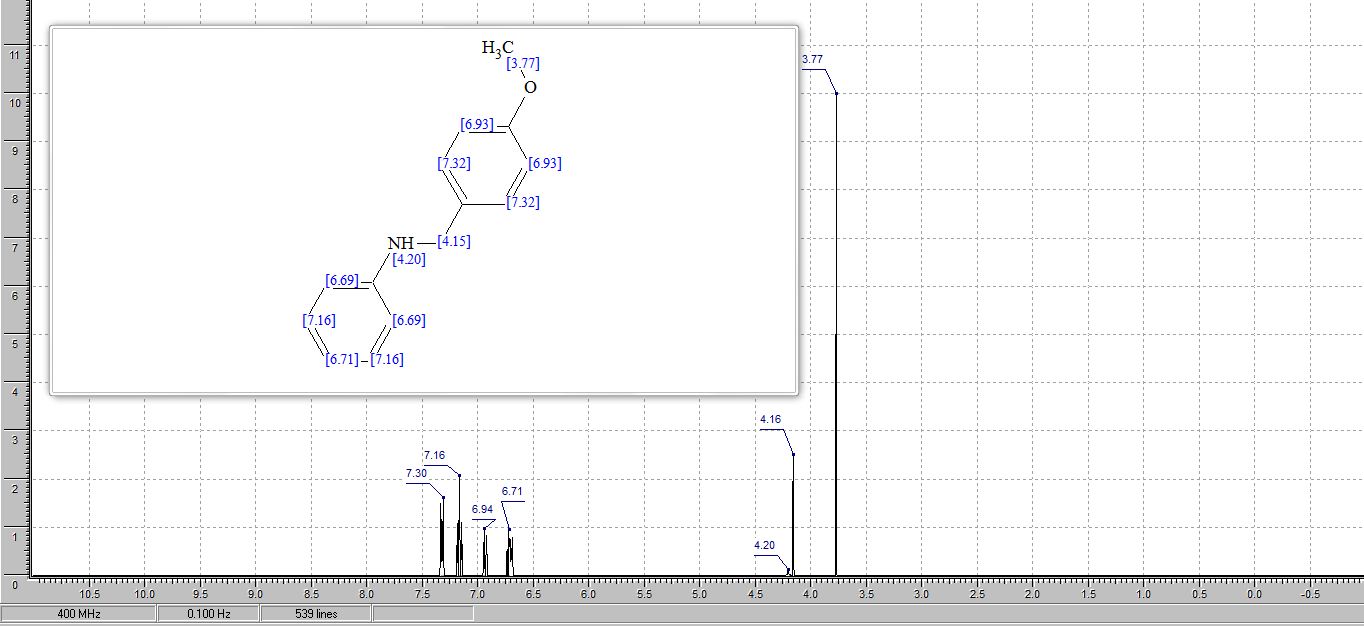
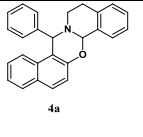
15-phenyl-7a,12,13,15-tetrahydronaphtho[1',2':5,6][1,3]oxazino[2,3- a]isoquinoline (4a):1White solid; Yield 61 %, 221 mg;
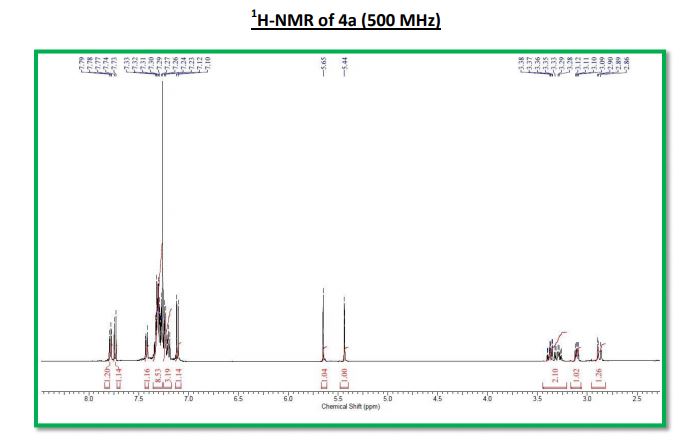
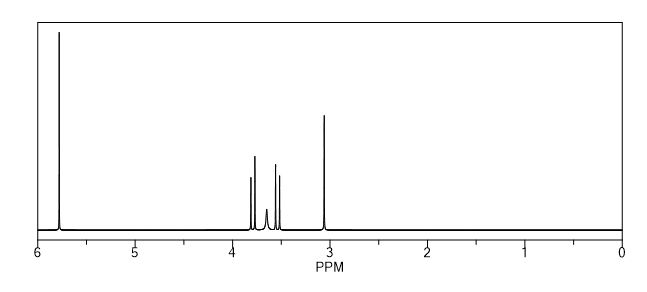
1H NMR (500 MHz, CDCl3): δ 7.79-7.77 (m, 1H), 7.74 (d, J = 8.9 Hz, 1H), 7.43-7.41 (m, 1H), 7.33-7.28 (m, 8H), 7.24-7.19 (m, 3H), 7.11 (d, J = 8.9 Hz, 1H), 5.65 (s, 1H), 5.44 (s, 1H), 3.40-3.26 (m, 2H), 3.12-3.09 (m, 1H), 2.90- 2.86 (m, 1H);
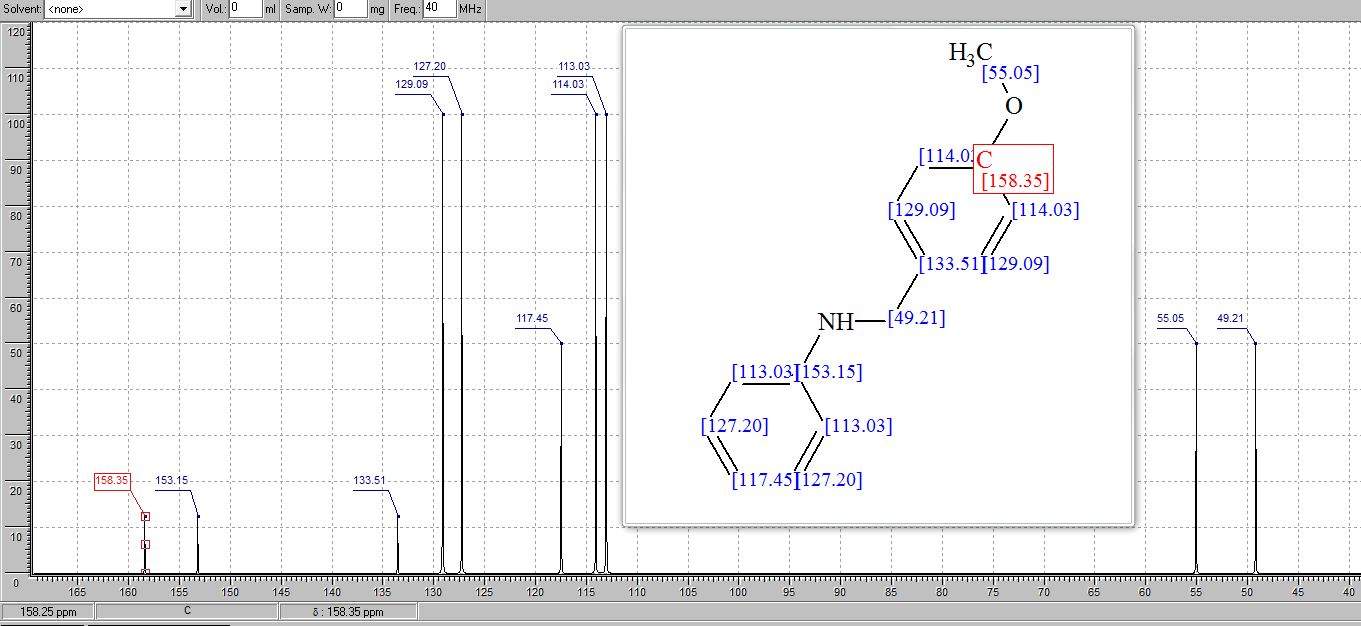
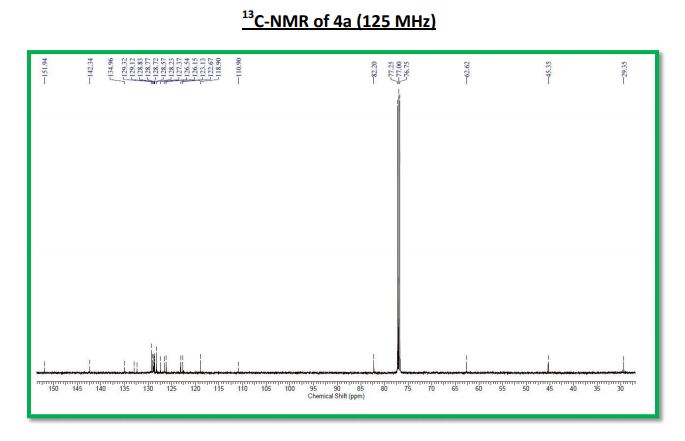
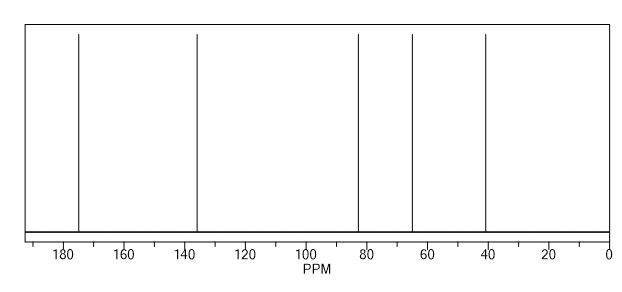
13C NMR (125 MHz, CDCl3): δ 151.9, 142.3, 135.0, 133.0, 132.4, 129.3, 129.1, 128.9, 128.8 (2C), 128.7, 128.6, 128.2, 127.4, 126.5, 126.2, 123.1, 122.7, 118.9, 110.9, 82.2, 62.6, 45.4, 29.4;
HRMS (ESI) exact mass calculated for C26H21NO [M+H]+ : 364.1701; found: 364.1705.
The representative procedure for the synthesis of 4a is as follows: 2-naphthol (1a, 144 mg, 1 mmol), benzaldehyde (2a, 106 mg, 1 mmol), tetrahydroisoquinoline (3, 133 mg, 1 mmol) and water (1.5 mL) were added in a round-bottom flask equipped with a magnetic stirring bar and a reflux condenser. The whole apparatus was efficiently flushed with oxygen gas and then connected to a balloon filled with oxygen. After vigorous stirring at 100 oC for 12 h, water was removed under vacuum and purified the reaction mixture by column chromatography (100-200 mesh silica gel, hexane-ethyl acetate) to obtain the product 4a as white solid. The other 1,3-oxazines were synthesized and purified by following the procedure described above
//////////////
 Open Access
Open Access