Enol form
| |

Keto form
| |
 | |
 |
Title: Curcumin
CAS Registry Number: 458-37-7
CAS Name: (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione
Additional Names: turmeric yellow; diferuloylmethane; C.I. 75300; C.I. Natural Yellow 3
Molecular Formula: C21H20O6
Molecular Weight: 368.38
Percent Composition: C 68.47%, H 5.47%, O 26.06%
Literature References: Natural dyestuff from root of Curcuma longa L., Zingiberaceae. Isoln: Vogel, Ann. 44, 297 (1842); Perkin, Phipps, J. Chem. Soc. (Trans.) 85, I, 64 (1904); Rao, Shintre, J. Soc. Chem. Ind. 47, 54T (1928). Synthesis: Lampe, Ber. 51,1347 (1918). Production: Stieglitz, Horn, DE 859145 (1952 to Hoechst). Biosynthesis studies: Roughley, Whiting, Tetrahedron Lett.1971, 3741. Chromatography: Srinivasan, J. Pharm. Pharmacol. 5, 448 (1953). See also: H. J. Conn's Biological Stains, R. D. Lillie, Ed. (Williams & Wilkins, Baltimore, 9th ed., 1977) pp 474-476. Pharmacology and anti-inflammatory activity: Srimal, Dhawan, ibid. 25, 447 (1973).
Properties: Orange-yellow, cryst powder, mp 183°. Insol in water, ether. Sol in alcohol, glacial acetic acid. Gives a brownish-red color with alkali; a light-yellow color with acids.
Melting point: mp 183°
Use: For preparing curcuma paper, pH range 8-9. In the detection of boron.
CurcuminCurcumin
(458-37-7)
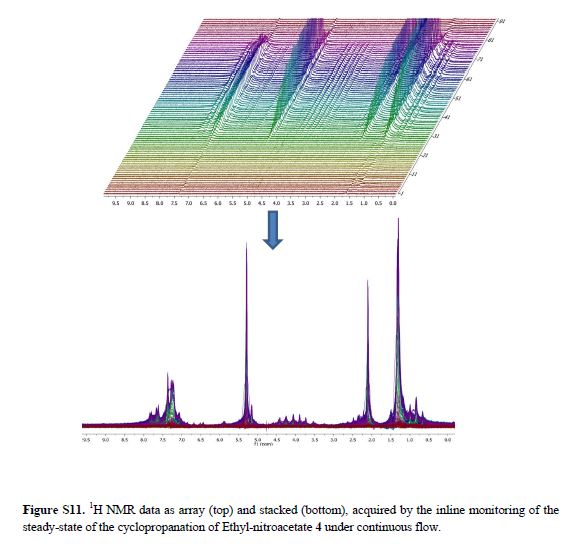
1HNMR
H NMR spectrum of C21H20O6 in CDCL3 at 400 MHz.
Shifts
| Index | Name | Shift (ppm) |
|---|---|---|
| 11 | HC6 | 7.392 |
| 24 | HC7 | 6.767 |
| 13 | HC1 | 7.583 |
| 15 | HC | 6.767 |
| 40 | HC10 | 6.989 |
| 33 | HC15 | 3.891 |
| 47 | HC5 | 6.989 |
| 42 | HC9 | 7.392 |
| 26 | HC8 | 7.583 |
| 35 | HC17 | 3.891 |
| 34 | HC16 | 3.891 |
| 1 | HC14 | 3.891 |
| 19 | HC2 | 4.058 |
| 3 | HC12 | 3.891 |
| 4 | HC13 | 3.891 |
| 8 | HC4 | 7.411 |
| 20 | HC3 | 4.058 |
| 29 | HC11 | 7.411 |
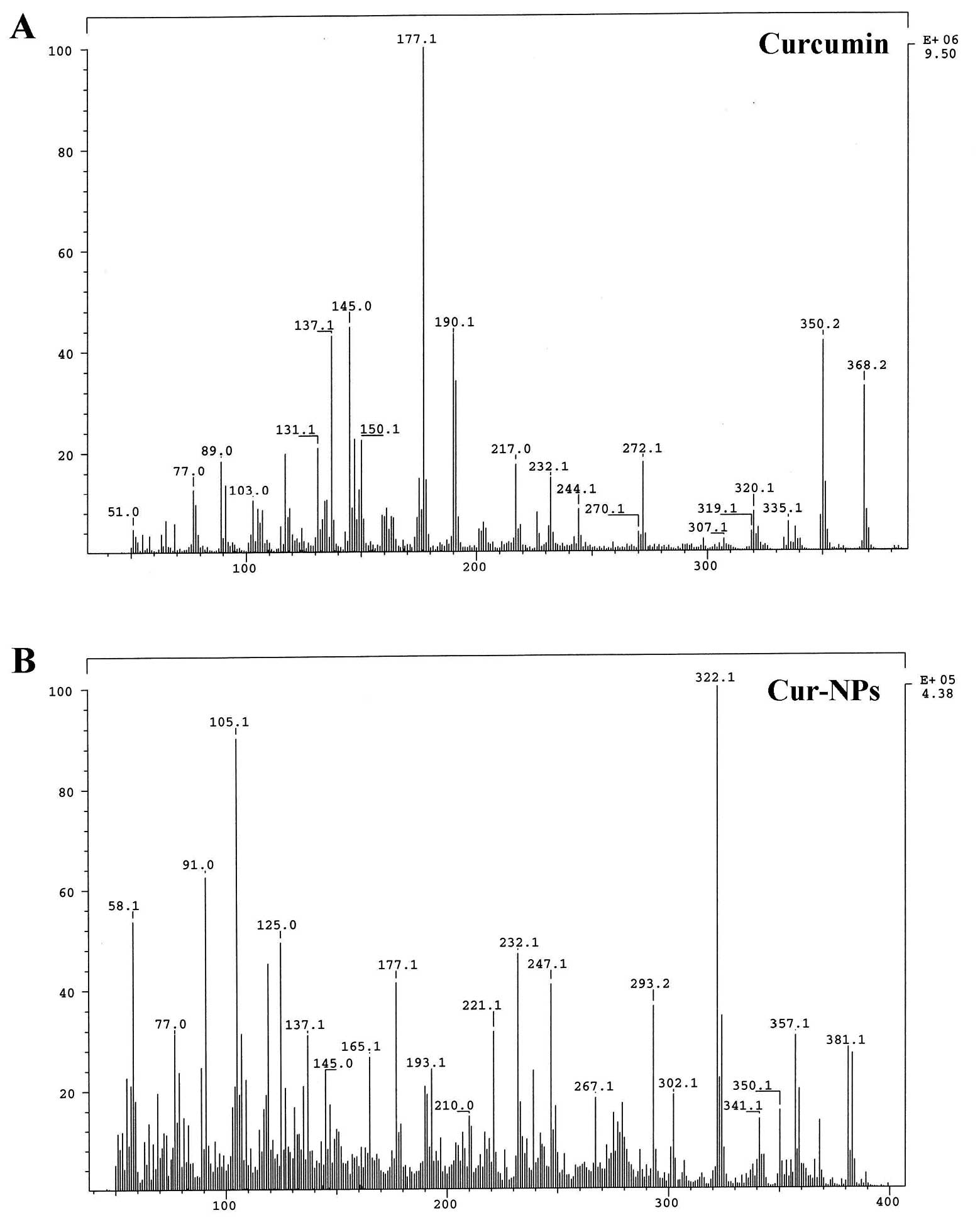
Figure 3. - Mass spectra of curcumin (A) and Cur-NPs (B). The mass spectra of curcumin and Cur-NPs were determined as described in Materials and methods.
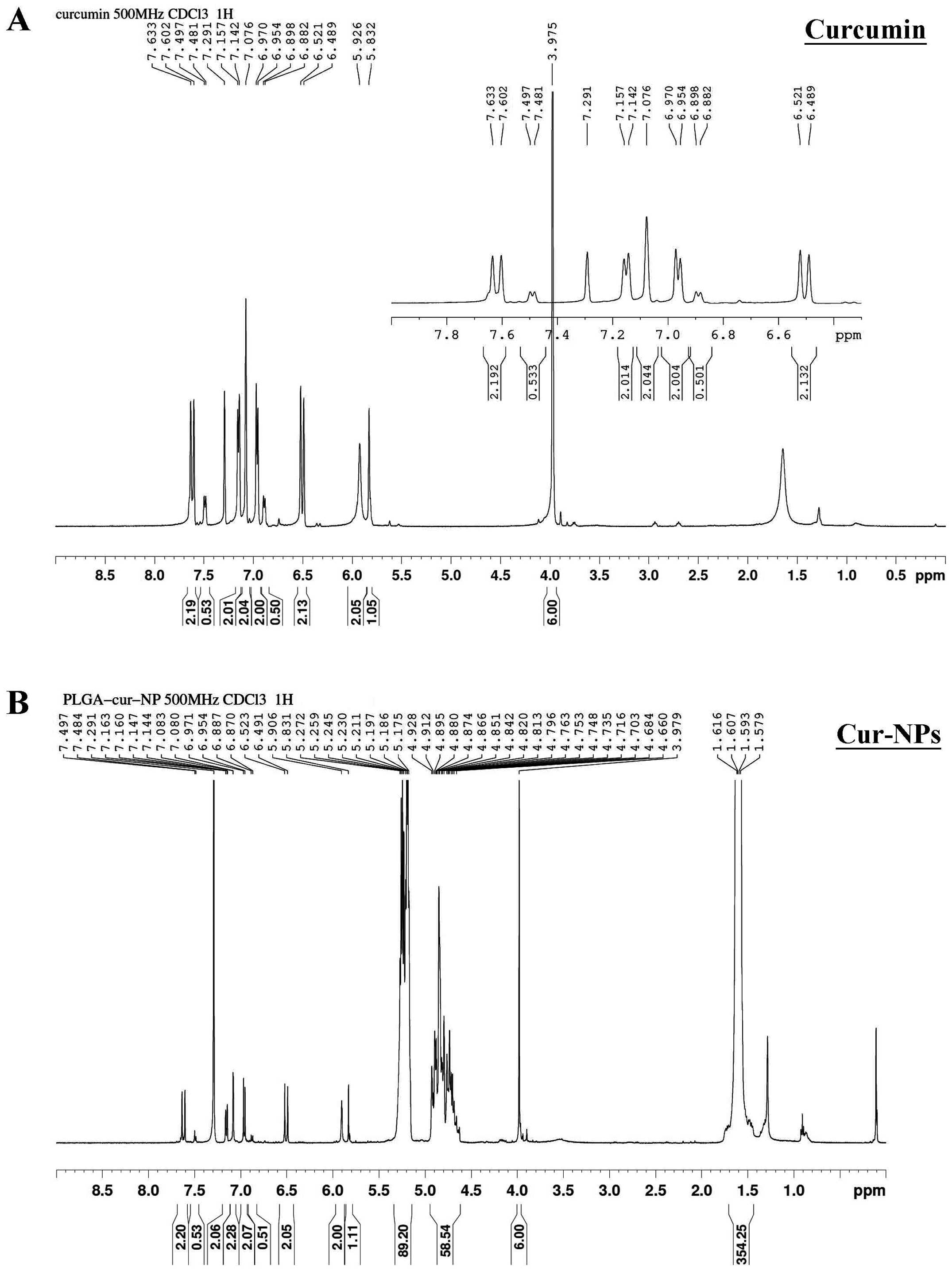
Figure 4. - 1H-NMR spectra of curcumin (A) and Cur-NPs (B). The 1H-NMR spectra of curcumin and Cur-NPs were determined as described in Materials and methods.

Curcumin A structure was verified by nuclear magnetic resonance analysis (1H-NMR) (CDCl3, 400 MHz, ppm) δ 7.66 (d, J =15.6 Hz, 2H), 7.18 (dd, J =8.0, 1.6 Hz, 2H), 7.11 (d, J =2 Hz, 2H), 6.96 (d, J =1.6 Hz, 2H), 6.91 (d, J =9.6 Hz, 2H), 5.96 (s, 2H), 3.95 (s, 6H)
https://www.dovepress.com/inhibition-of-hiv-1-by-curcumin-a-a-novel-curcumin-analog-peer-reviewed-fulltext-article-DDDT

https://www.researchgate.net/publication/6490144_NMR_Study_of_the_Solution_Structure_of_Curcumin
NMR Study of the Solution Structure of Curcumin Article in Journal of Natural Products · March 2007 DOI: 10.1021/np060263s · Source: PubMed
http://iopscience.iop.org/article/10.1088/1757-899X/107/1/012063/pdf
SEE
Revisiting Curcumin Chemistry Part I: A New Strategy for the Synthesis ...
www.ncbi.nlm.nih.gov › NCBI › Literature › PubMed Central (PMC)
by EV Rao - 2011 - Cited by 9 - Related articles
A new strategy for the synthesis of curcuminoids is described involving the reaction of acetylacetone difluroboronite with an aromatic aldehyde in the presence of ...[PDF]The Chemistry of Curcumin: From Extraction to Therapeutic Agent
www.mdpi.com/1420-3049/19/12/20091/pdf
by KI Priyadarsini - 2014 - Cited by 42 - Related articles
Dec 1, 2014 - analytical chemists. In organic chemistry the extraction and synthesis of curcumin and new synthetic derivatives was the main focus of research.Isolation and synthesis of curcumin - ResearchGate
https://www.researchgate.net/file.PostFileLoader.html?id...assetKey...
May 31, 2012 - To obtain pure curcumin, column chromatography was needed ... steps fromcurcumin analogues, the synthesis was a faster way of obtaining ...http://oasys2.confex.com/acs/236nm/techprogram/P1189704.HTM
http://www.mdpi.com/1420-3049/19/12/20091/htm
https://openi.nlm.nih.gov/detailedresult.php?img=PMC2674881_1471-2407-9-99-1&req=4
- Curcumin
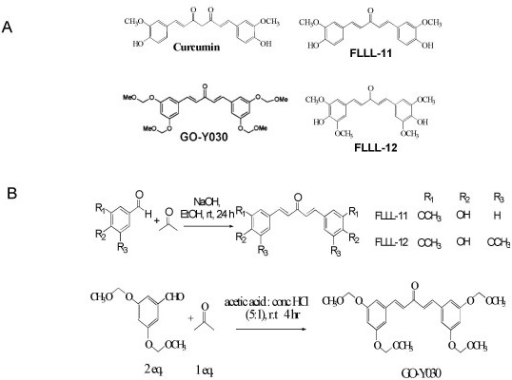
Synthesis of curcumin was first described by Lampe et al. In our laboratory curcumin has been synthesized by condensing vanillin (I) and acetyl acetone (II) in a medium of ethyl acetate using tributylborate as boron complex to avoid Knoevenagel condensation at C-3 of acetyl acetone. Curcumin is isolated from the reaction mixture by acidification and extraction with ethyl acetate. The organic layers are washed until neutral, dried and the solvent is removed. purified by chromatography over silica gel using ether/petroleum ether as the solvent.
Scrimal, R.C.; Curcumin. Drugs Fut 1987, 12, 4, 331- Curcumin
The protection of 4-bromo-2-methoxyphenol (I) with ethyl vinyl ether (II) and TsOH in dichloromethane gives the ethoxyethyl ether (III), which is treated with n-BuLi in THF to yield the phenyl lithium compound (IV). The reaction of (IV) with 2H-labeled DMF (V), followed by hydrolysis with HCl, affords the labeled 4-hydroxy-3-methoxybenzaldehyde (VI), which is finally condensed with pentane-2,4-dione (VII) by means of B2O3 and tetrahydroquinoline in DMF.
Threadgill, M.D.; Parveen, I.; Labelled compounds of interest as antitumour agents - VII. [H-2]- and [C-14]-curcumin. J Label Compd Radiopharm 2000, 43, 9, 883
Curcumin
The protection of 4-bromo-2-methoxyphenol (I) with ethyl vinyl ether (II) and TsOH in dichloromethane gives the ethoxyethyl ether (III), which is treated with n-BuLi in THF to yield the phenyl lithium compound (IV). The reaction of (IV) with 14C-labeled DMF (V), followed by hydrolysis with HCl, affords the labeled 4-hydroxy-3-methoxybenzaldehyde (VI), which is finally condensed with pentane-2,4-dione (VII) by means of B2O3 and tetrahydroquinoline in DMF.
Threadgill, M.D.; Parveen, I.; Labelled compounds of interest as antitumour agents - VII. [H-2]- and [C-14]-curcumin. J Label Compd Radiopharm 2000, 43, 9, 883

https://www.researchgate.net/publication/224918513_Curcumin-From_Molecule_to_Biological_Function/figures?lo=1
| Curcumin | |
| Scrimal, R.C. | |
| Drugs Fut 1987,12(4),331 | |
Synthesis of curcumin was first described by Lampe et al. In our laboratory curcumin has been synthesized by condensing vanillin (I) and acetyl acetone (II) in a medium of ethyl acetate using tributylborate as boron complex to avoid Knoevenagel condensation at C-3 of acetyl acetone. Curcumin is isolated from the reaction mixture by acidification and extraction with ethyl acetate. The organic layers are washed until neutral, dried and the solvent is removed. purified by chromatography over silica gel using ether/petroleum ether as the solvent.
| |
| Labelled compounds of interest as antitumour agents - VII. [H-2]- and [C-14]-curcumin | |
| Threadgill, M.D.; Parveen, I. | |
| J Label Compd Radiopharm 2000,43(9),883 | |
The protection of 4-bromo-2-methoxyphenol (I) with ethyl vinyl ether (II) and TsOH in dichloromethane gives the ethoxyethyl ether (III), which is treated with n-BuLi in THF to yield the phenyl lithium compound (IV). The reaction of (IV) with 2H-labeled DMF (V), followed by hydrolysis with HCl, affords the labeled 4-hydroxy-3-methoxybenzaldehyde (VI), which is finally condensed with pentane-2,4-dione (VII) by means of B2O3 and tetrahydroquinoline in DMF.
| |
The protection of 4-bromo-2-methoxyphenol (I) with ethyl vinyl ether (II) and TsOH in dichloromethane gives the ethoxyethyl ether (III), which is treated with n-BuLi in THF to yield the phenyl lithium compound (IV). The reaction of (IV) with 14C-labeled DMF (V), followed by hydrolysis with HCl, affords the labeled 4-hydroxy-3-methoxybenzaldehyde (VI), which is finally condensed with pentane-2,4-dione (VII) by means of B2O3 and tetrahydroquinoline in DMF.
| |
//////////