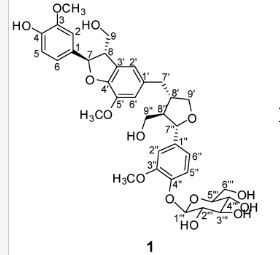
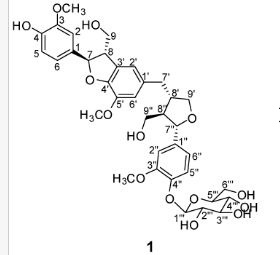
(7R, 8S, 8′S, 7″S, 8″R)-abiesol A 4″-O-β-d-glucopyranoside, and it was named capselloside.
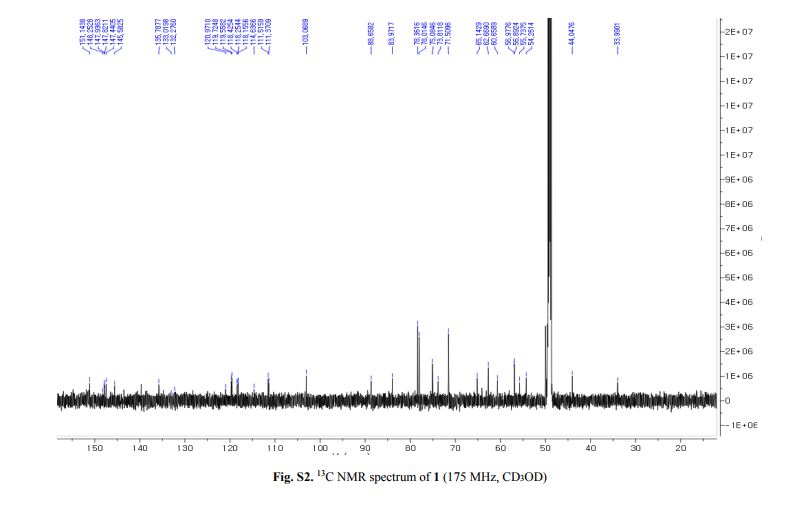
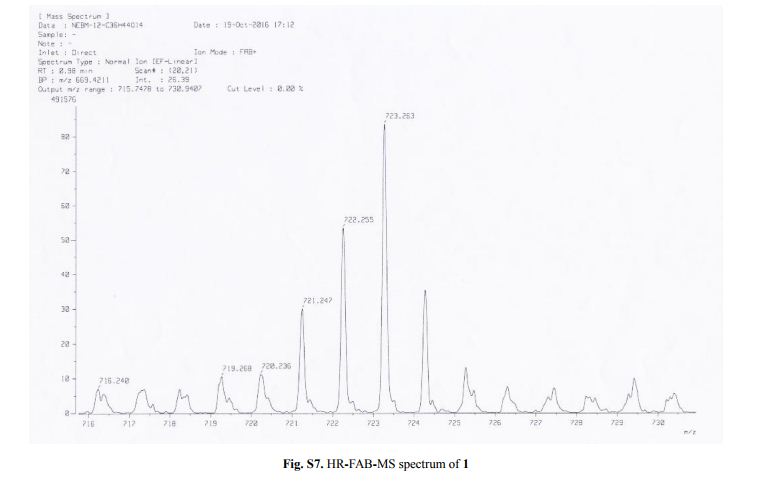
Capselloside (1): Brownish gum; [α]25D −34.0 (c = 0.05, MeOH); UV (MeOH) λmax (log ε) 280 (1.4), 228 (3.3), 214 (3.1) nm; IR (KBr) νmax 3261, 2946, 2816, 1638, 1489, 1261 cm−1; CD (MeOH) λmax (Δ ε) 291 (−), 280 (−) 257 (−) 230 (−) 223 (+); 1H (700 MHz) and 13C (175 MHz) NMR data, see ; HR-FABMS (positive-ion mode) m/z: 723.2626 [M + Na]+ (calcd. for C36H44O14Na, 723.2629).
Compound 1 was obtained as a brownish gum. The molecular formula was established as C36H44O14using HR-FABMS, which showed a positive ion [M + Na]+ at m/z: 723.2626 (calcd. for C36H44O14Na, 723.2629, so the molecular formula was deduced to be C36H44O14.
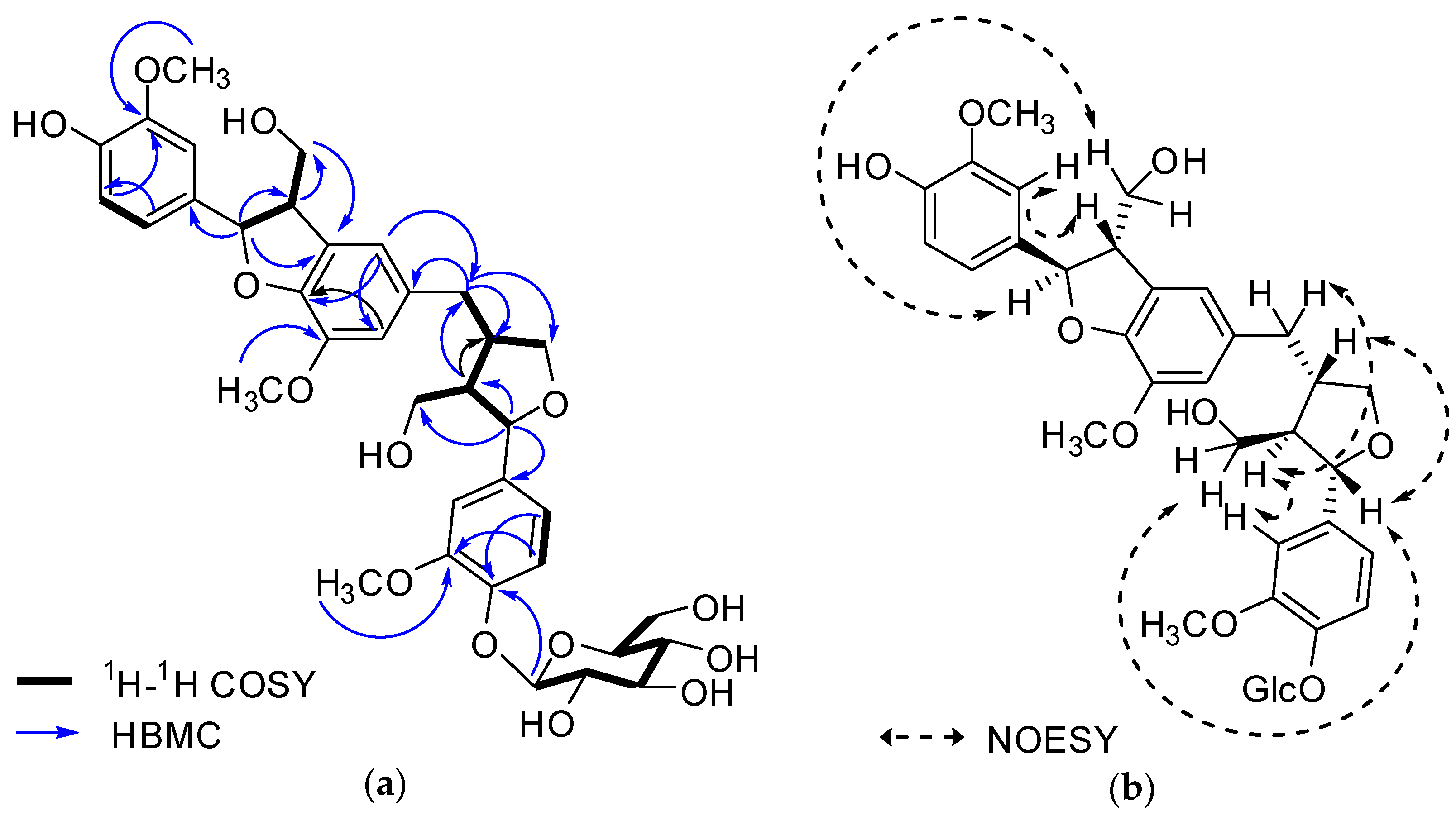
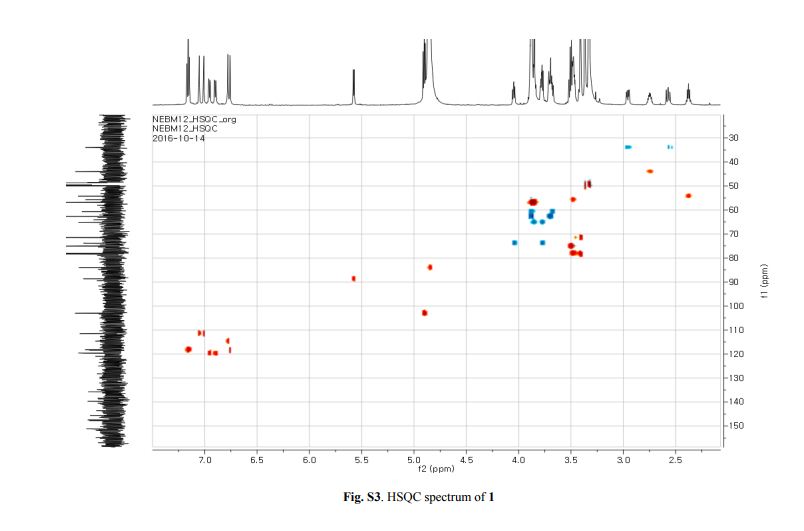
The 1H-NMR spectrum of 1 revealed the presence of two 1,3,4-trisubstituted aromatic rings (δH 7.16 (d, J = 8.3 Hz, H-5′′), 7.15 (d, J = 8.2 Hz, H-5), 7.05 (d, J = 1.7 Hz, H-2), 7.01 (d, J = 1.7 Hz, H-2′′), 6.95 (dd, J = 8.2, 1.7 Hz, H-6), and 6.90 (dd, J = 8.2, 1.7 Hz, H-6″)), a 1,3,4,5-tetrasubstitued aromatic ring (δH 6.76 (s, H-6′), 6.78 (s, H-2′)), two oxygenated methines (δH 5.58 (d, J = 5.9 Hz, H-7) and 4.85 (m, H-7′′)), two oxygenated methylenes (δH 3.85 (m, H-9b), 3.78 (m, H-9a), and 3.88 (m, H-9′′a), 3.68 (dd, J = 10.9, 6.7 Hz, H-9b′′)), a methylene 2.96 ((dd, J = 13.5, 5.0 Hz, H-7′a), and 2.57 (dd, J = 13.3, 11.0 Hz, H-7′b)), three methines (δH 3.48 (m, H-8), 2.75 (sep, J = 6.5, 5.5 Hz, H-8′), and 2.38 (quin, J = 6.9 Hz, H-8″)), three methoxy groups (δH 3.88 (s, 3-OCH3), 3.87 (s, 5′-OCH3) and 3.84 (s, 3′′-OCH3)), and a glucopyanosyl unit (δH 4.91 (d, J = 7.3 Hz, H-1′′′), 3.69 (m, H-6′′′), 3.51 (m, H-2′′′), 3.41 (overlap, H-4′′′, 5′′′) and 3.39 (m, H-3′′′).
The 13C-NMR spectrum displayed 36 carbon signals, including 18 aromatic carbons δC 151.1 (C-3″), 147.9 (C-4′, 3′), 147.8 (C-3), 147.5 (C-4, 4″), 145.5 (C-5′), 139.7 (C-1″), 135.7 (C-1), 134.9 (C-1′), 119.7 (C-6″), 119.5 (C-6), 118.4 (C-6′), 118.2 (C-5″), 118.1 (C-5), 114.7 (C-2′), 111.5 (C-2″) and 111.3 (C-2), three oxygenated carbons δC 88.6 (C-7), 83.9 (C-7″), and 73.5 (C-9′), three methylene carbons δC 65.1 (C-9), 60.5 (C-9″), 33.9 (C-7′) and three methine carbons δC55.7 (C-8), 54.2 (C-8″), 44.0 (C-8′), and, three methoxy carbons (δC 56.9 and 56.8 (×2)), and glucose carbons δc 103.1 (C-1′′′), 78.5 (C-3′′′), 78.4 (C-5′′′), 75.1 (C-2′′′), 71.6 (C-4′′′), and 62.6 (C-6′′′) ().
Table 1. 1H (700 MHz) and 13C (175 MHz) NMR data of 1 in methanol-d4 (δ in ppm) a.
| Position | δC, Type | δH (J in Hz) |
|---|
| 1 | 135.7, C | |
| 2 | 111.3, CH | 7.05, d (1.7) |
| 3 | 147.8, C | |
| 4 | 147.5, C | |
| 5 | 118.1, CH | 7.15, d (8.2) |
| 6 | 119.5, CH | 6.95, dd (8.2, 1.7) |
| 7 | 88.6, CH | 5.58, d (5.91) |
| 8 | 55.7, CH | 3.48, m |
| 9 | 65.1, CH2 | |
| a | | 3.78, m |
| b | | 3.85, m |
| 1′ | 134.9, C | |
| 2′ | 114.7, CH | 6.78, s |
| 3′ | 147.9, C | |
| 4′ | 147.9, C | |
| 5′ | 145.5, C | |
| 6′ | 118.4, CH | 6.76, s |
| 7′ | 33.9, CH2 | |
| a | | 2.96, dd (13.5, 5.0) |
| b | | 2.57, dd (13.3, 11.0) |
| 8′ | 44.0, CH | 2.75, sep (6.5, 5.5) |
| 9′ | 73.5, CH2 | |
| a | | 3.78, m |
| b | | 4.05, dd (8.3, 6.6) |
| 1″ | 139.7, C | |
| 2″ | 111.5, CH | 7.01, d (1.7) |
| 3″ | 151.1, C | |
| 4″ | 147.5, C | |
| 5″ | 118.2, CH | 7.16, d (8.3) |
| 6″ | 119.7, CH | 6.90, dd (8.2, 1.7) |
| 7″ | 83.9, CH | 4.85, overlap |
| 8″ | 54.2, CH | 2.38, quin (6.9) |
| 9″ | 60.5, CH2 | |
| a | | 3.88, m |
| b | | 3.68, dd (10.9, 6.7) |
| Glc-1′′′ | 103.1, CH | 4.91, d (7.3) |
| 2′′′ | 75.1, CH | 3.51, m |
| 3′′′ | 78.5, CH | 3.39, m |
| 4′′′ | 71.6, CH | 3.41, m |
| 5′′′ | 78.4, CH | 3.41, m |
| 6′′′ | 62.6, CH | 3.69, overlap |
| OCH3 (3″) | 56.9 | 3.84, s |
| OCH3 (3) | 56.8 | 3.88, s |
| OCH3 (5′) | 56.8 | 3.87, s |
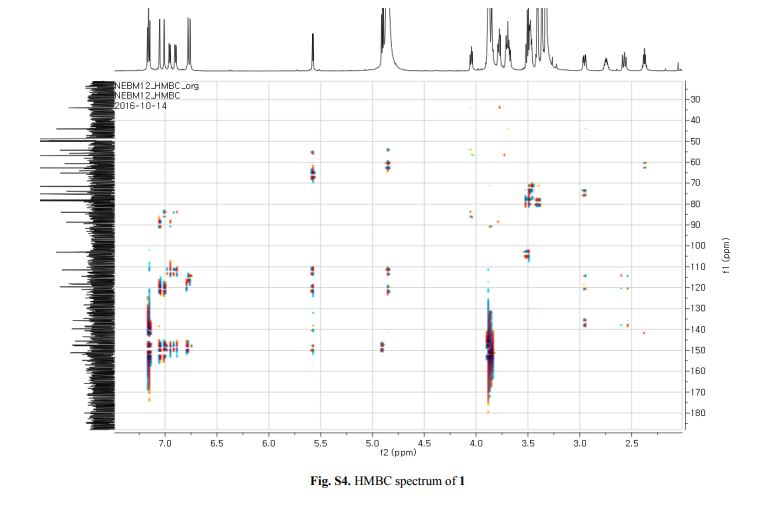
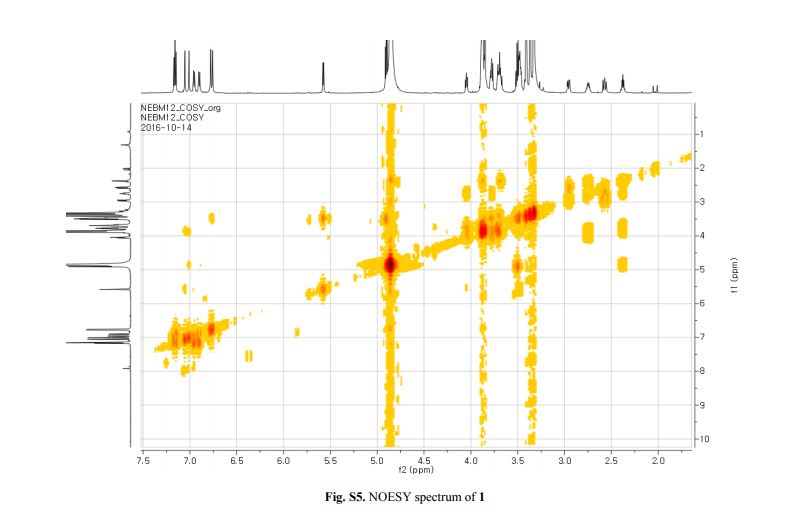
Figure 2. Key 1H-1H COSY, HMBC (a) and NOESY (b) correlations of 1.
Article
Phenolic Glycosides from Capsella bursa-pastoris (L.) Medik and Their Anti-Inflammatory Activity
Joon Min Cha 1, Won Se Suh 1, Tae Hyun Lee 1, Lalita Subedi 2,3, Sun Yeou Kim 2,3 and Kang Ro Lee 1,*
1
Natural Products Laboratory, School of Pharmacy, Sungkyunkwan University, Suwon 16419, Korea
2
Gachon Institute of Pharmaceutical Science, Gachon University, 191 Hambakmoero, Yeonsu-gu, Incheon 21936, Korea
3
College of Pharmacy, Gachon University, 191 Hambakmoero, Yeonsu-gu, Incheon 21936, Korea
*
Correspondence: Tel.: +82-31-290-7710; Fax: +82-31-290-7730
Received: 16 May 2017 / Accepted: 18 June 2017 / Published: 20 June 2017

Abstract:
A new sesquilignan glycoside 1, together with seven known phenolic glycosides 2–8 were isolated from the aerial parts of Capsella bursa-pastoris. The chemical structure of the new compound 1 was elucidated by extensive nuclear magnetic resonance (NMR) data (1H- and 13C-NMR, 1H-1H correlation spectroscopy (1H-1H COSY), heteronuclear single-quantum correlation (HSQC), heteronuclear multiple bond correlation (HMBC), and nuclear overhauser effect spectroscopy (NOESY)) and HR-FABMS analysis. The anti-inflammatory effects of 1–8 were evaluated in lipopolysaccharide (LPS)-stimulated murine microglia BV-2 cells. Compounds 4 and 7 exhibited moderate inhibitory effects on nitric oxide production in LPS-activated BV-2 cells, with IC50 values of 17.80 and 27.91 µM, respectively.
Keywords:
Capsella bursa-pastoris; Cruciferae; sesquilignan glycoside; anti-inflammatory
/////////capselloside, nmr, cosy













![2D [1H,13C]-HSQC](http://mmcd.nmrfam.wisc.edu/png/expnmr_00689_6.png)
![2D [1H,13C]-HMBC](http://mmcd.nmrfam.wisc.edu/png/expnmr_00689_7.png)
![2D [1H,1H]-COSY](http://mmcd.nmrfam.wisc.edu/png/expnmr_00689_8.png)