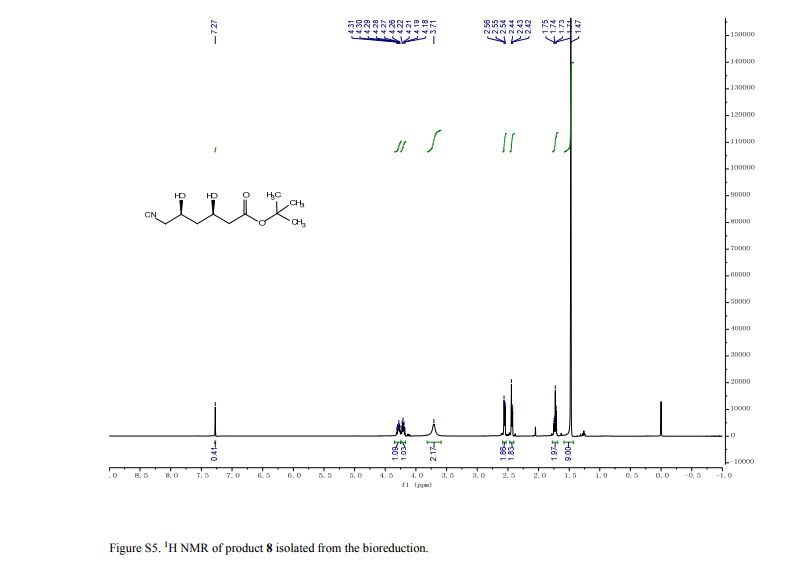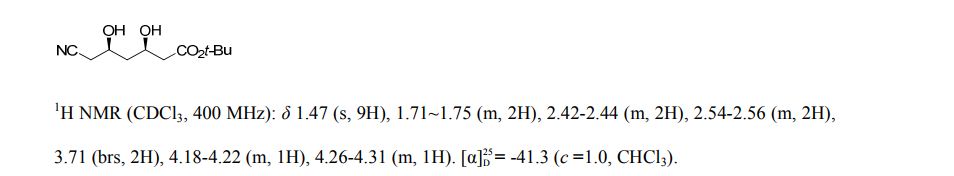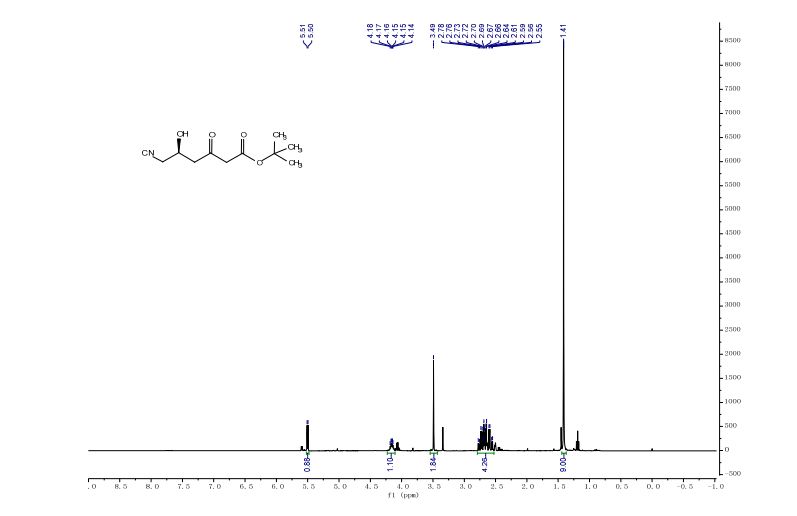
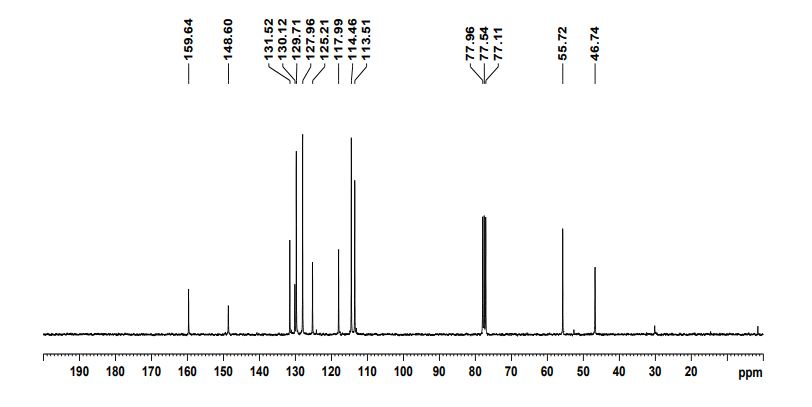
N‒(4’‒methoxyCinnamyl)aniline
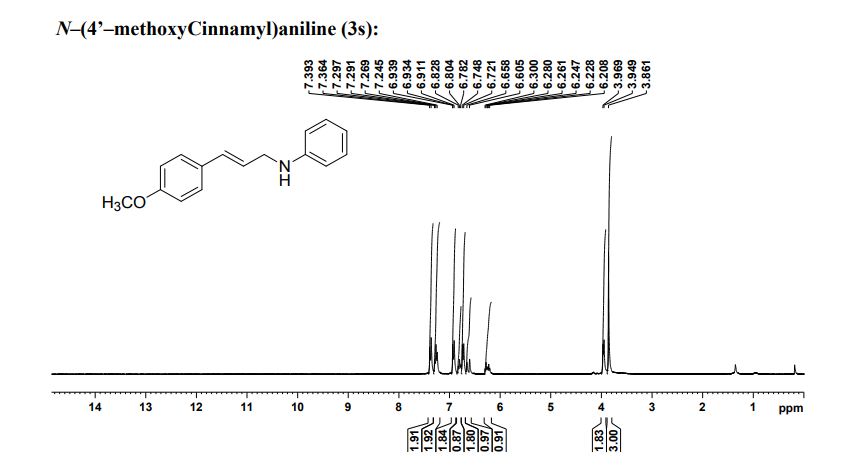
N‒(4'‒Methoxycinnamyl)aniline (3s): Yellow brown solid (203 mg, 85% yield),
mp. 75 oC,
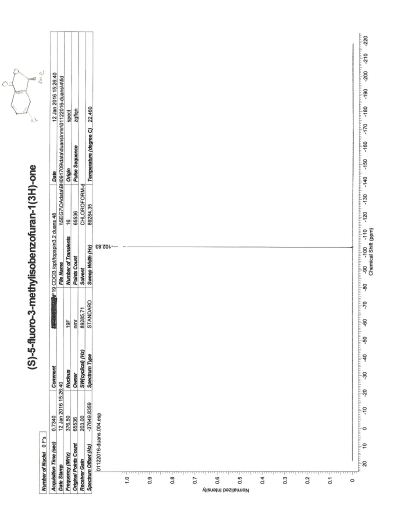
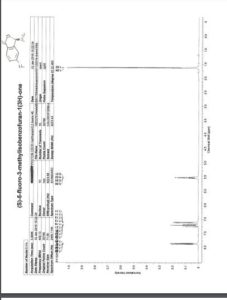
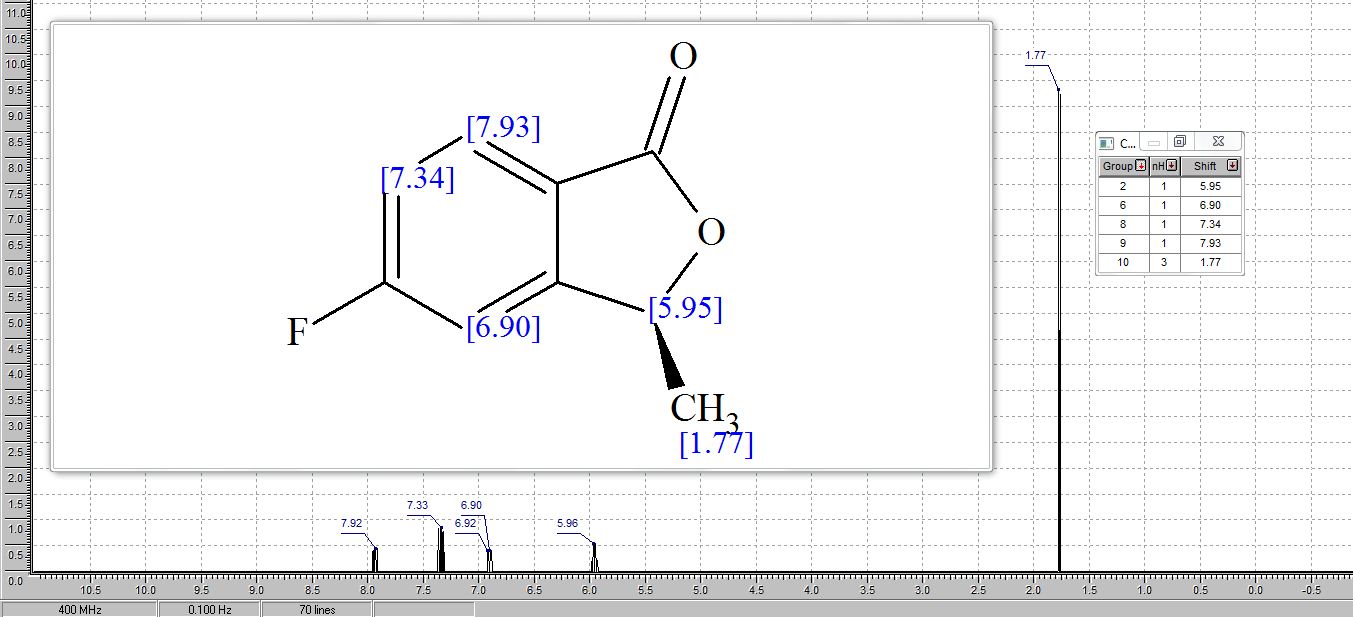
1H NMR (CDCl3, 600 MHz): δ 3.86 (s, 3H), 3.94 (d, 2H, J = 11.5 Hz), 6.20‒ 6.29 (m, 1H), 6.60 (d, 1H, J = 15.9 Hz), 6.74 (d, 2H, J = 8.28 Hz), 6.80 (t, 1H, J = 6.9 Hz), 6.93 (d, 2H, J = 4.32 Hz), 7.24‒7.29 (m, 2H), 7.38 (d, 2H, J = 8.6 Hz);
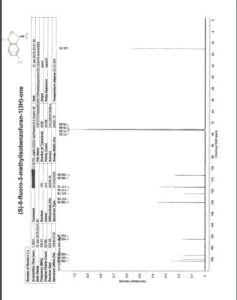
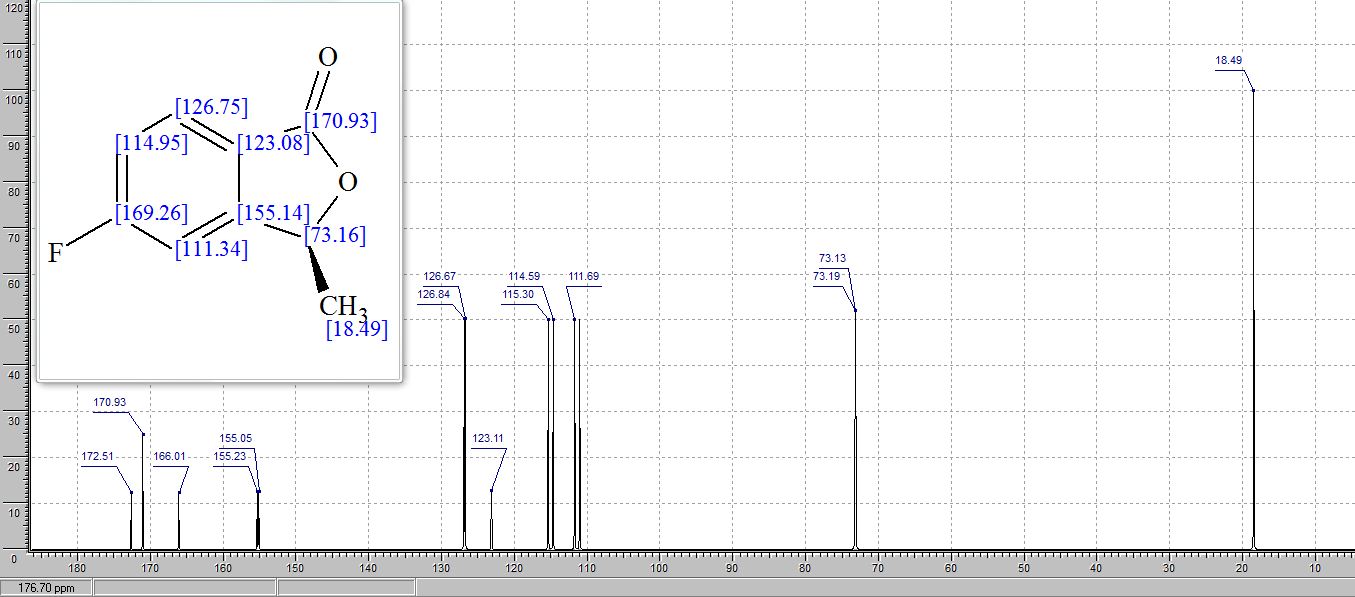
13C NMR (CDCl3, 150 MHz): δ 46.7, 55.7, 113.5, 114.4, 117.9, 125.2, 127.9, 129.7, 130.1, 131.5, 148.6, 159.6;
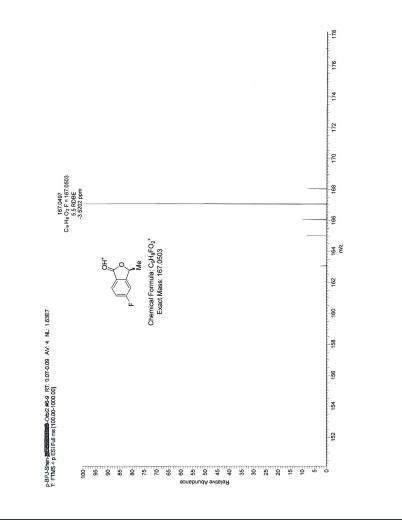
HRESIMS calcd for C16H18NO [M+H]+ 240.1388, found 240.1378.
EVEN MOM CAN TEACH U NMR
.gif?id=10359&width=137)

//////////













![2D [1H,13C]-HSQC](http://mmcd.nmrfam.wisc.edu/png/expnmr_00689_6.png)
![2D [1H,13C]-HMBC](http://mmcd.nmrfam.wisc.edu/png/expnmr_00689_7.png)
![2D [1H,1H]-COSY](http://mmcd.nmrfam.wisc.edu/png/expnmr_00689_8.png)