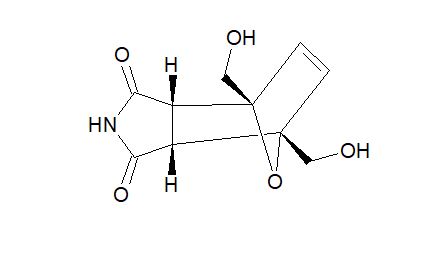
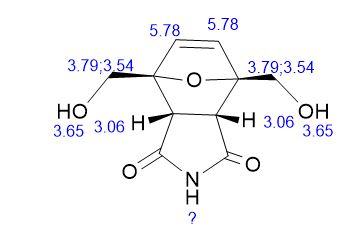
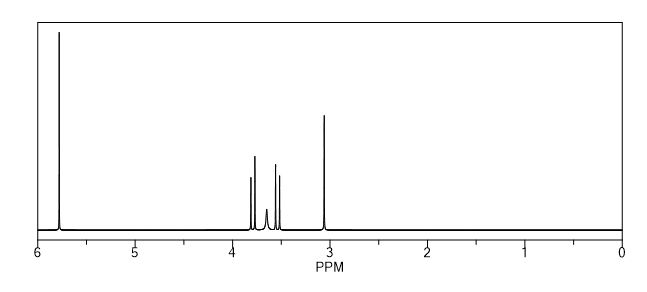
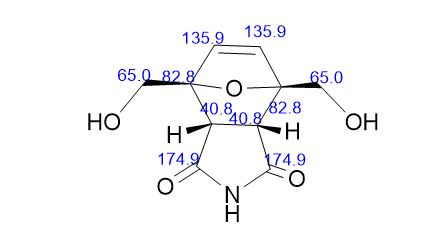
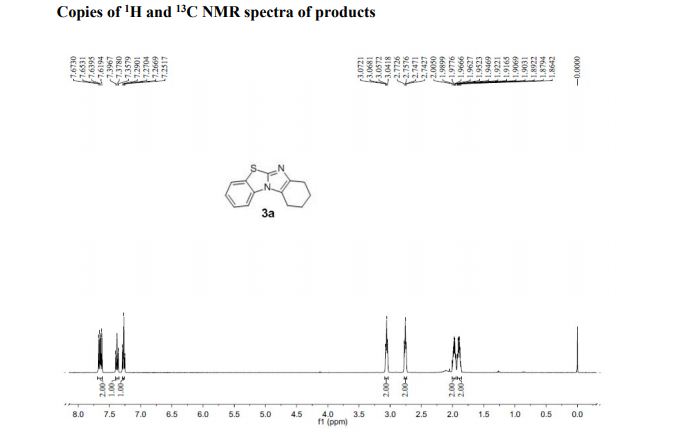
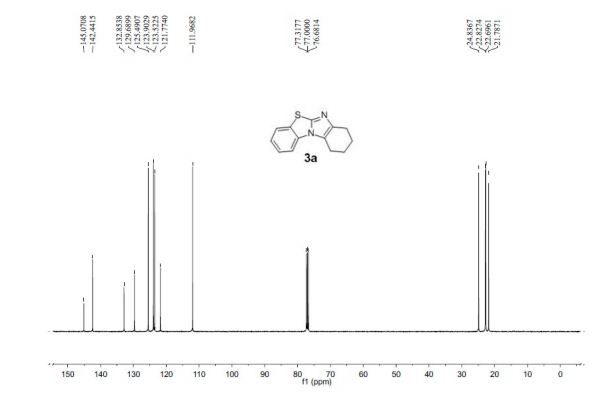
2,5-Diformylfuran (DFF), 5 (lit. 2
) 2 Kashparova, V. P., Khokhlova, E. A., Galkin, K. I., Chernyshev, V. M. & Ananikov, V. P. The “onepot”
synthesis of 2,5-diformylfuran, a promising synthon for organic materials in the conversion
of biomass. Russ. Chem. Bull. 64, 1069-1073 (2015).
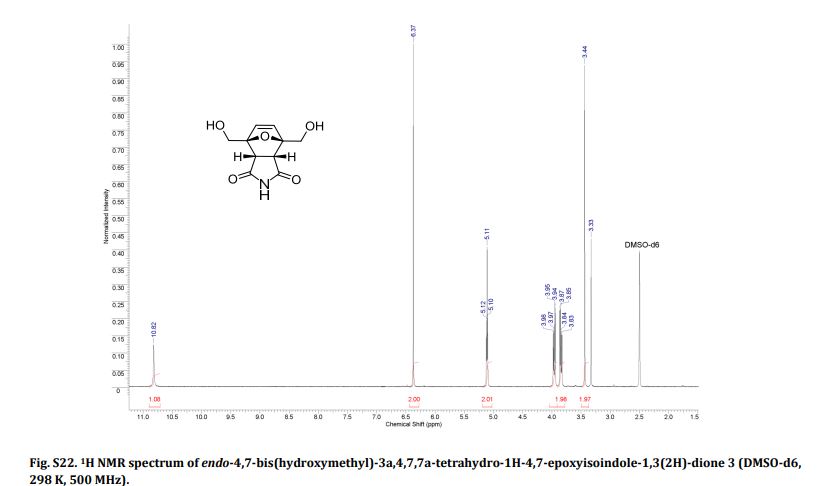
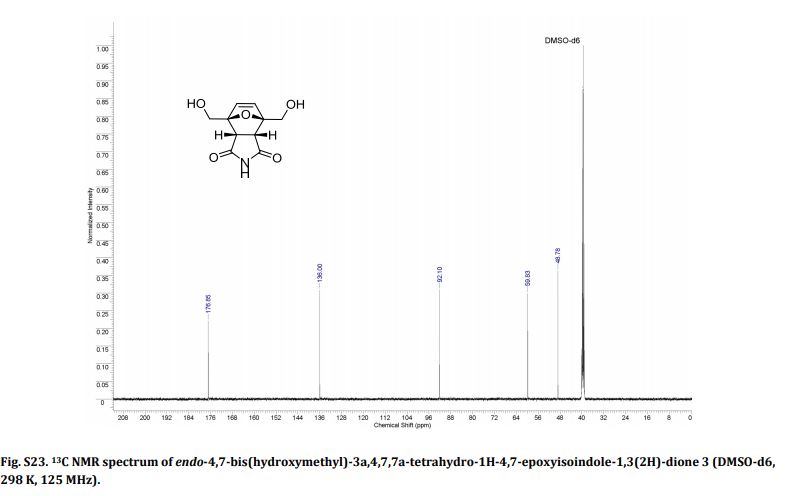
1H NMR (CDCl3) = 9.87 (s, 2H), 7.35 (s, 2H);
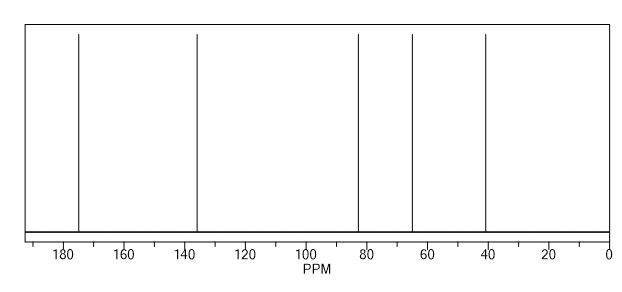
13C NMR (CDCl3) = 181.1, 154.1, 122.5 ppm.
//////////
1H NMR (CDCl3) = 9.87 (s, 2H), 7.35 (s, 2H);
13C NMR (CDCl3) = 181.1, 154.1, 122.5 ppm.
Green Chem., 2017, Advance Article
DOI: 10.1039/C7GC02211E, Paper
DOI: 10.1039/C7GC02211E, Paper
F. A. Kucherov, K. I. Galkin, E. G. Gordeev, V. P. Ananikov
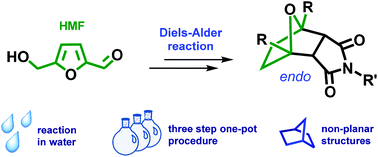
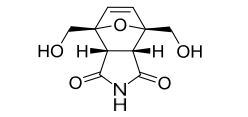
Efficient one-pot synthesis of tricyclic compounds from biobased 5-hydroxymethylfurfural (HMF) is described using a [4 + 2] cycloaddition reaction.
Efficient one-pot synthesis of tricyclic compounds from biobased 5-hydroxymethylfurfural (HMF) is described using a [4 + 2] cycloaddition reaction.