

NMR.http://file.selleckchem.com/downloads/nmr/S208601-Ivabradine-hydrochloride-NMR-Selleck.pdf......SEEMS ORIGINAL
1H NMR PREDICTION OF BASE
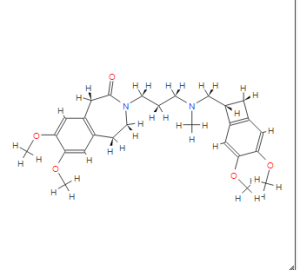
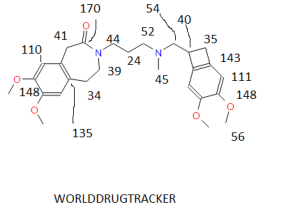
CAS NO. 155974-00-8, 3-[3-[[(7S)-3,4-dimethoxy-7-bicyclo[4.2.0]octa-1,3,5-trienyl]methyl-methylamino]propyl]-7,8-dimethoxy-2,5-dihydro-1H-3-benzazepin-4-one
![3-[3-[[(7S)-3,4-dimethoxy-7-bicyclo[4.2.0]octa-1,3,5-trienyl]methyl-methylamino]propyl]-7,8-dimethoxy-2,5-dihydro-1H-3-benzazepin-4-one NMR spectra analysis, Chemical CAS NO. 155974-00-8 NMR spectral analysis, 3-[3-[[(7S)-3,4-dimethoxy-7-bicyclo[4.2.0]octa-1,3,5-trienyl]methyl-methylamino]propyl]-7,8-dimethoxy-2,5-dihydro-1H-3-benzazepin-4-one H-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2015-01-17/001/562/1562114_1h.png)
13 C NMR PREDICT
![3-[3-[[(7S)-3,4-dimethoxy-7-bicyclo[4.2.0]octa-1,3,5-trienyl]methyl-methylamino]propyl]-7,8-dimethoxy-2,5-dihydro-1H-3-benzazepin-4-one NMR spectra analysis, Chemical CAS NO. 155974-00-8 NMR spectral analysis, 3-[3-[[(7S)-3,4-dimethoxy-7-bicyclo[4.2.0]octa-1,3,5-trienyl]methyl-methylamino]propyl]-7,8-dimethoxy-2,5-dihydro-1H-3-benzazepin-4-one C-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2015-01-17/001/562/1562114_13c.png)
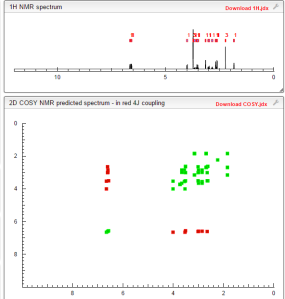
COSY PREDICTIONS
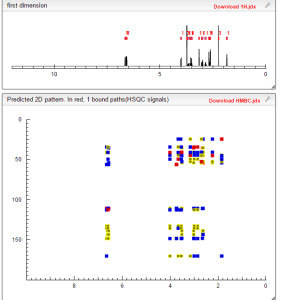
HMBC PREDICTIONS
Amgen has received approval from the US Food and Drug Administration (FDA) for its Corlanor (ivabradine) to treat patients with chronic heart failure.
Corlanor is an oral medication indicated to reduce the risk of hospitalisation for worsening heart failure in patients with stable and symptomatic chronic heart failure with left ventricular ejection fraction (LVEF).
Developed by Les Laboratoires Servier, Amgen holds rights to commercialise Corlanor in the US, through collaboration with Servier.
Amgen research and development executive vice-president Dr Sean Harper said: "We are excited to introduce Corlanor, the first new chronic heart failure medicine approved by the FDA in nearly a decade, for patients who are at a significantly greater risk of hospitalisation due to worsening heart failure in the US.
"We hope that today's approval of Corlanor as an innovative therapeutic option will address a major unmet need for patients, their families and the healthcare system."

"We hope that approval of Corlanor as a therapeutic option will address a major unmet need for patients, their families and the healthcare system."
Corlanor is said to block hyperpolarisation-activated cyclic nucleotide-gated (HCN) channel responsible for the cardiac pacemaker that regulates heart rate.
It will reduce the spontaneous pacemaker activity of the cardiac sinus node by selectively inhibiting the If-current to slow the heart-rate with no effect on ventricular repolarisation and no impact on myocardial contractility.
Approval was based on global clinical trial data, including a large, multi-centre, randomised, double-blind, placebo-controlled and outcomes trial.
The Phase III Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT) compared Corlanor against placebo on top of standard of care (SOC) therapies, including beta blockers in around 6,500 clinically stable patients.
According to the company, Phase III SHIFT study demonstrated that Corlanor significantly reduced the risk of primary composite endpoint of hospitalisation or cardiovascular death for worsening heart failure.
...............................................
US2014/107334
the ivabradine hydrochloride is obtained by the process of the instant disclosure by following the process of scheme 1 below:
Example 4: Preparation of Ivabradine base (Formula-7)
3- {3-[(3,4-dimethoxy-bicyclo[4.2.0]octa-l ,3,5-trien-7-ylmethyl0-methyl-amino]- propyl} -7,8-dimethoxy-l ,3-dihydro-benzo[d]azepin-2-one (Formula-6) (17.5g, 0.0375mol.) is added with 87.5ml of acetic acid. To the mass, 3.5g of 20%> Palladium hydroxide (dry) is added and hydrogenated under hydrogen pressure of 3 - 4Kg/cm2 at 25 - 30°C for 3hr.
The mass is filtered through celite bed. Filtrate is concentrated to 1.4w/w under reduced pressure at 50 - 55°C. To the residual mass. 87.5ml of ethyl acetate is added and the product is extracted using water twice (87.5ml and 52.5ml). The combined aqueous layer is basified using Sodium hydroxide and the product is extracted using ethyl acetate thrice (87.5ml, 2 x 52.5ml). The combined organic layer is washed with water and then concentrated under vacuum at 45 - 50°C to get about 14.94g (85%) of Ivabradine base (Formula-7) having the purity >75%
Example 5:
Preparation of Ivabradine Hydrochloride (Formula-1)
To the Ivabradine base (Formula-7) (15g, 0.032mol.), 37.5ml. of IN hydrochloric acid is added. The mass is stirred for lhr and filtered through celite bed. Filtrate is concentrated to about 1.2w/w under reduced pressure at 55 - 60°C. To the residual mass, acetonitrile is added and stripped off to about 1.2w/w thrice (3 x 30ml). 75ml of acetonitrile is added and stirred the mass for lhr at 78 - 82°C. The mass was cooled to 25 - 29°C and stirred for lhr. The mass is filtered and the wet material is washed with acetonitrile. Wet material was dried under vacuum at 85 - 95°C to get 9.7g (60%>) of Ivabradine Hydrochloride (Formula 1)
3- {3-[(3,4-dimethoxy-bicyclo[4.2.0]octa-l ,3,5-trien-7-ylmethyl0-methyl-amino]- propyl} -7,8-dimethoxy-l ,3-dihydro-benzo[d]azepin-2-one (Formula-6) (17.5g, 0.0375mol.) is added with 87.5ml of acetic acid. To the mass, 3.5g of 20%> Palladium hydroxide (dry) is added and hydrogenated under hydrogen pressure of 3 - 4Kg/cm2 at 25 - 30°C for 3hr.
The mass is filtered through celite bed. Filtrate is concentrated to 1.4w/w under reduced pressure at 50 - 55°C. To the residual mass. 87.5ml of ethyl acetate is added and the product is extracted using water twice (87.5ml and 52.5ml). The combined aqueous layer is basified using Sodium hydroxide and the product is extracted using ethyl acetate thrice (87.5ml, 2 x 52.5ml). The combined organic layer is washed with water and then concentrated under vacuum at 45 - 50°C to get about 14.94g (85%) of Ivabradine base (Formula-7) having the purity >75%
Example 5:
Preparation of Ivabradine Hydrochloride (Formula-1)
To the Ivabradine base (Formula-7) (15g, 0.032mol.), 37.5ml. of IN hydrochloric acid is added. The mass is stirred for lhr and filtered through celite bed. Filtrate is concentrated to about 1.2w/w under reduced pressure at 55 - 60°C. To the residual mass, acetonitrile is added and stripped off to about 1.2w/w thrice (3 x 30ml). 75ml of acetonitrile is added and stirred the mass for lhr at 78 - 82°C. The mass was cooled to 25 - 29°C and stirred for lhr. The mass is filtered and the wet material is washed with acetonitrile. Wet material was dried under vacuum at 85 - 95°C to get 9.7g (60%>) of Ivabradine Hydrochloride (Formula 1)
............................
WO2011138625
Process for the synthesis of Ivabradine salts of formula (I) the chemical name of which is: 3-{3-[((S)-3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-triene- 7-ylmethyl)-methyl-amino]-propyl}-7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepine-2-one salts (HQ=HCl, HBr, HI, HNO3, HClO4, (COOH)2),
Ivabradine of formula (I) is an alternative drug for the treatment of stable angina pectoris in case of intolerance or contraindication for beta receptor blockers.
The first synthesis of Ivabradine of formula (I) is described in EP 534859 Patent of Servier.
According to this process the iodopropyl-benzazepinone derivative intermediate of formula (XI) is reacted with ((lS)-3,4-dimethoxy-bicyclo[4.2.0]octa-l,3,5-triene-7-yl-methyl)- methyl-amine of formula (Vlllb) and the obtained product of formula (Xb) furthermore dehydro-Ivabradine is hydrogenated.
According to the known methods, described e.g. in: US4490369 the reactive iodopropyl-benzazepinone derivative of formula (XI) is synthesized starting from l-(7,8-dimethoxy-l,3-dihydro-2H-3-benzazepine-2-on-3-yl)-3-chloro-propane reacting with sodium iodide and isolated in cr stalline form.

Ivabradine
US 7176197 B2 Patent of Servier describes the synthesis of Ivabradine of formula (I) and the key intermediate is amino hydrochloride of formula (VIII).
According to this process Ivabradine of formula (I) is prepared by reductive alkylation in the presence of hydrogen and Pd/C catalyst starting form the compound of formula (VIII) with a protected aldehyde e.g.: 3-(2-[l,3]-dioxolan-2-yl-ethyl)-7,8-dimethoxy-l,3-dihydro-2H-3-benzazepine-2-one in one step. Paralelly, the dihydro-benzazepine moiety is saturated to tetrahydro-benzazepine structure in during the process.

Hydrochloride salt of Ivabradine
WO 2008 65681 PCT of Cadila describes an alternative synthetic method of Ivabradine of formula (I).
The key intermediate of this process is the methylamino-methyl-cyclobutane derivative of formula (VHIb) which is reacted with l -bromo-3-chloro-propane to the tertiary amine of formula (XII). Then the potassium salt of lactam of formula (XIII) iss alkylated with the obtained chloro-propyl-methyl-amine derivative.
According e.g.: to US4490369 Patent azetidine ring can also be produced under the conditions of alkylation of the chloropropyl tertiary amine of formula (XII).

The key intermediate of the above processes is the enantiomer pure ((S)-3,4-dimethoxy-bicyclo[4.2.0]octa-l,3,5-triene-7-yl-methyl)-methyl-amine of formula (Vlllb).
EP 534859 Patent describes the preparation of this compound in enantiomer pure form. According to this process the compound of formula (Vlllb) is prepared from the racemic form by resolution with d-camphorsulfonic acid.
The camphorsulfonic salt is purified by repeated recrystallizations until the ee purity of 99% was obtained.
According to their examples the ratio of S/R enantiomers is only 84/16 and the yield is only 40%.
WO 2005 123659 PCT Patent Application of Servier describes a novel process for the preparation of enantiomer pure methylamino-methyl-benzocyclobutane derivative of formula (VIII).


The racemic aminomethyl-benzocyclobutane derivative of formula (XlVrac) is prepared by reduction of the nitrile compound of formula (III).
The diastereomeric salt is obtained by resolution of the racemic amine by N-acetyl-L-glutamic acid then the salt is purified by recrystallization.
The intermediate of formula (XV) is prepared from the enantiomeric pure compound of formula (XIV) and the formed urethane of formula (XV) is reduced by lithium-aluminum-hydride (LAH).
The yield of the resolution is increased to ca.: 35-40%, the used resolving agent is cheaper than d-camphoric acid, however the 60 % of the racemic amine (XlVrac) is lost during the resolution, since the (R)-aminomethyl-benzocyclobutane is not regenerated.
The key intermediate of Ivabradine: ((5)-3,4-dimethoxy-bicyclo[4.2.0]octa-l ,3,5-triene-7-yl-methyl)-methyl-amine compound of formula (VHIb) and the salts thereof are prepared according to the above processes, mainly starting from the carbonitrile compound of formula (III). The synthesis of the compound of formula (III) can be carried out on different ways.
According to e.g.: US 4618683 Patent the starting material is 3-(2-bromo-4,5-dimethoxyphenyl)-propionitrile. Flammable and moisture sensitive alkali metal amide (e.g.: sodium or potassium) is used for the reaction in liquid ammonia at low temperature (generally at reflux temperature of ammonia: -33 °C).

In the publication of Tetrahedron Letters, Vol.23, No.36, 3669-3672, 1982 study the flash vacuum pyrolysis (F.V.P.) of 2-methyl-benzyl-chlorides is described. In the examples the preparation of the nitrile compound of formula (III) is also described. The 2-methyl-4,5-dimethoxybenzaldehyde compound of formula (XVI) is reacted with the corrosive phosphorus pentachloride to yield the benzylidene-dichloride compound of formula (XVII). The dimethoxy-benzocyclobutane compound of formula (XVIII) was obtained by pyrolysis of compound of formula (XVII) at low pressure (0.1 mbar) and at high temperature with 60 % yield. The key intermediate of formula (III) is obtained by substitution the chloro atom with ciano group using tetraalkyl-ammonium-cianide (R4NCN). The yield is 38 % yield.
(XVI) (XVII) (XVIII)
US 2010 16580A1 Patent Application of Servier describes the preparation of benzocyclobutane derivatives. In their process the bromophenyl-malonic ester-derivative of formula (XIX) is cyclized to diester of formula (XX) by palladium catalysis with 69% yield.
The diester of formula (XX) is purified by chromatography and then treated with trifluoro acetic acid followed by decarboxylation to yield dimethoxy-benzocyclobutenoic ester of formula (XXI).
This ester is reacted with aqueous solution of methylamine to form the amide of formula (Vllrac). This amide of formula (Vllrac) is reduced with borane/THF complex and the formed secondary amine of formula (VHIrac) is isolated as the hydrochloride salt thereof.
The obtained secondary amine is resolved with camphorsulfonic acid and enantiomer pure amine of formula (Vlllb) was formed.

(Vllrac) (Vlllrac)
Only a few inorganic or organic salts of Ivabradine (I) are known. The hydrochloride salt is described in Servier's EP 534859 Patent.
Servier published more Patents which claim polymorphs and hydrate forms of Ivabradine (WO 2005/110993, WO 2006/092491, WO 2006/092492, WO 2006/092493, WO 2006/092494, WO 2007/042656, WO 2007/042657).
According to these, Ivabradine HCl exists in the form of at least one stable anhydrate and three different hydrates and the same metastable dehydrated hydrate forms.
Further known Ivabradine salts are the hydrobromide (WO 2009/124940) and oxalate (WO 2008/146308).
reaction scheme.

......................
.......................................
CN101768116
European Patent EP 0534859 have been described in the preparation of ivabradine and its hydrochloride,
Its route is as follows:
The disadvantage is that the method of obtaining ivabradine required by column chromatography, and the reaction yield is very low, above all limited its industrialization.
Another patent CN200510051779.0 China reported a new method for the synthesis of ivabradine, the route is as follows:
The two-step reaction route use the precious metal palladium on carbon as catalyst, and are using the autoclave reaction, although the total yield is higher, but the cost and industrialization considerations disadvantages of this route.
DETAILED DESCRIPTION
Example 1: 3_ (3-hydroxypropyl) -7,8-dimethoxy-4,5-dihydro -1H- benzazepine -2 (3H) - one
2.3g3- (3- hydroxypropyl) -7,8-dimethoxy--1H- benzazepine _2 (3H) - one and 1.8g 10% Pd / C was added to 20mL of methanol, and then to the system was gradually introduced into H2, inside the system was bubbled uniform. Heating system so that the reaction at 40 ° C under 24h. Recovered by suction filtration Pd / C, and the filtrate rotary evaporated to give a white solid 1.97g, yield: 85%, mp 102.5 ° C. IH-NMR (O) Cl3,400MHz), δ 1.72-1.75 (t, 2H), 3.05-3.08 (t, 2H), 3.48-3.51 (t, 2H), 3.58-3.61 (t, 2H),
3.71-3.74 (q, 2H), 3.84-3.85 (d, 8H), 6.57-6.60 (d, 2H). NMR see attached Figure 1
2 Embodiment: ivabradine hydrochloride preparation
0.4g 3- (3- hydroxypropyl) -7,8-dimethoxy-4,5-dihydro -1H- benzazepine -2 (3H) - one and
0.3g (S) - (4, 5- dimethoxy-1,2-dihydrobenzofuran-cyclobutyl-1-yl) -N- methylmethanamine was added to IOmL in acetone, and then added 0.2 g KI and 0.5g K2CO3, start stirring and heated to reflux. After 24h the reaction the reaction was stopped.Filtration, the filtrate was spin-dries sticky solid which was dispersed in water, the filter cake was suction filtered, and the filtrate was discarded. Then dissolved in ethyl acetate, washed with 3N HCl solution, and then reconcile with sodium hydroxide solution to a pH 7-8, dried over anhydrous magnesium sulfate, and rotary evaporated under reduced pressure to give an oil. The oil was dispersed in ethyl acetate as the monohydrochloride, to give 0.5g of white crystalline powder was recrystallized from methanol and acetone in 75% yield, m.p. 196.30C ο 1H-Nmr (D2OAOOMHz), δ 2.15 (s, 2H), 2.66-2.70 (d, 2H),
2.91 (s, 3H), 3.00-3.03 (t, 2H), 3.12-3.14 (t, 2H), 3.16-3.18 (t, IH), 3.23-3.29 (t, 1H),
3.41-3.44 (t, 1H), 3.56-3.60 (t, 1H), 3.62-3.66 (d, 6H), 3.68-3.75 (m, 3H), 3.81-3.84 (d, 6H), 3.86-3.90 (q , 2H), 3.93-3.96 (t, 1Η), 6.64-6.80 (q, 4Η), nuclear magnetic map See Figure 2.
Figure 1: 3_ (3-hydroxypropyl) -7,8-dimethoxy-4,5-dihydro -1H- benzazepine -2 (3H) - one, NMR chart,
Figure 2: ivabradine hydrochloride, NMR chart.
| CN101284813A | 12 Apr 2007 | 15 Oct 2008 | 上海优拓医药科技有限公司;常州市第四制药厂有限公司 | Preparation method of ivabradine | |
| CN101434552A | 19 May 2008 | 20 May 2009 | 江苏恒瑞医药股份有限公司 | Method for splitting 4,5- dimethoxy-1-(methyl amino methyl)-benzocyclobutane | |
| CN101768116A | 29 Dec 2008 | 7 Jul 2010 | 北京德众万全药物技术开发有限公司 | Preparation method for Ivabradine | |
| WO2008/065681A2 | Title not available |
| CN1683341A | 18 Feb 2005 | 19 Oct 2005 | 瑟维尔实验室 | New process for the synthesis of ivabradine and addition salts thereof with a pharmaceutically acceptable acid |
| CN1948293A | 11 Oct 2006 | 18 Apr 2007 | 瑟维尔实验室 | Delta d-crystalline form of ivabradine hydrochloride, a process for its preparation and pharmaceutical compositions containing it |
| CN101284813A | 12 Apr 2007 | 15 Oct 2008 | 上海优拓医药科技有限公司;常州市第四制药厂有限公司 | Preparation method of ivabradine |
| US5296482 | 25 Sep 1992 | 22 Mar 1994 | Adir Et Compagnie | (Benzocycloalkyl) alkylamines |
| CN101723897B | 31 Oct 2008 | 16 Nov 2011 | 齐鲁制药有限公司 | Method for synthesizing Ivabradine |
| CN101768116B | 29 Dec 2008 | 18 Jun 2014 | 北京德众万全药物技术开发有限公司 | Preparation method for Ivabradine |
| CN101768117B | 29 Dec 2008 | 7 May 2014 | 北京德众万全药物技术开发有限公司 | 一种盐酸伊伐布雷定晶型及其制备方法 |
| CN101851205A * | 31 Mar 2010 | 6 Oct 2010 | 瑟维尔实验室 | New process for the synthesis of ivabradine and addition salts thereof with a pharmaceutically acceptable acid |
| CN101851205B | 31 Mar 2010 | 13 Feb 2013 | 瑟维尔实验室 | New process for the synthesis of ivabradine and addition salts thereof with a pharmaceutically acceptable acid |
| CN102603634B | 31 Mar 2010 | 16 Apr 2014 | 瑟维尔实验室 | Process for the synthesis of ivabradine and its pharmaceutically acceptable acid addition salts |
| CN102827080B | 12 Sep 2012 | 19 Mar 2014 | 江苏宇田生物医药科技有限公司 | Novel synthetic method of ivabradine and novel intermediate product of ivabradine |
| CN103232360B | 16 Feb 2011 | 21 May 2014 | 瑟维尔实验室 | 合成伊伐布雷定及其可药用酸加成盐的方法 |
| CN1683341A | 18 Feb 2005 | 19 Oct 2005 | 瑟维尔实验室 | New process for the synthesis of ivabradine and addition salts thereof with a pharmaceutically acceptable acid |
| CN101284813A | 12 Apr 2007 | 15 Oct 2008 | 上海优拓医药科技有限公司;常州市第四制药厂有限公司 | Preparation method of ivabradine |
| US5296482 | 25 Sep 1992 | 22 Mar 1994 | Adir Et Compagnie | (Benzocycloalkyl) alkylamines |
| Cited Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| WO2008146308A2 * | 29 May 2008 | 4 Dec 2008 | Ind Swift Lab Ltd | Process for the preparation of ivabradine hydrochloride and polymorph thereof |
| WO2013102919A1 * | 12 Nov 2012 | 11 Jul 2013 | Cadila Healthcare Limited | Polymorphic forms of ivabradine hydrochloride |
| Reference | ||
|---|---|---|
| 1 | * | HAI, L. ET AL.: 'Synthesis of 3-(3-chloropropyl)-1,3,4,5-tetrahydro-7,8-d imethoayl-2H-3- benzazepin-2-one' WEST CHINA JOURNAL OF PHARMACEUTICAL SCIENCES. vol. 24, 2009, pages 471 - 472 |
| 2 | * | LIANG, H.-Y. ET AL.: 'Synthesis and biological activity of some 1,3-dihydro-2H-3- benzazepin-2-ones with a piperazine moiety as bradycardic agents' ARCHIV DER PHARMAZIE - CHEMISTRY IN LIFE SCIENCES. vol. 343, 2010, pages 114 - 119 |
| 3 | * | LU, J. ET AL.: 'Polymorphism and crystallization of active pharmaceutical ingredients (APIs)' CURRENT MEDICINAL CHEMISTRY. vol. 16, 2009, pages 884 - 905 |
Reference:
LES LABORATOIRES SERVIER Patent: US2011/201805 A1, 2011 ; Location in patent: Page/Page column 3 ;
Les Laboratoires Servier; Renaud, Jean-Luc; Pannetier, Nicolas; Lecouve, Jean-Pierre; Vaysse-Ludot, Lucile; Moulin, Solenne Patent: US8513410 B2, 2013 ; Location in patent: Page/Page column 9; 10 ;
LES LABORATOIRES SERVIER; LE FLOHIC, Alexandre; GRANDJEAN, Mathieu Patent: US2013/261298 A1, 2013 ; Location in patent: Paragraph 0103; 0104; 0105 ;
LES LABORATOIRES SERVIER Patent: US2012/172589 A1, 2012 ; Location in patent: Page/Page column 3 ;
US2014/107334 A1,
| Systematic (IUPAC) name | |
|---|---|
| 3-[3-({[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methyl}(methyl)amino)propyl]-7,8-dimethoxy-2,3,4,5-tetrahydro-1H-3-benzazepin-2-one | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Licence data | EMA:Link |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration | Oral |
| Pharmacokinetic data | |
| Bioavailability | 40% |
| Protein binding | 70% |
| Metabolism | Hepatic (first-pass) >50%,CYP3A4-mediated |
| Half-life | 2 hours |
| Excretion | Renal and fecal |
| Identifiers | |
| CAS Registry Number | 155974-00-8 |
| ATC code | C01EB17 |
| PubChem | CID 132999 |
| IUPHAR ligand | 2357 |
| ChemSpider | 117373 |
| UNII | 3H48L0LPZQ |
| KEGG | D07165 |
| ChEMBL | CHEMBL471737 |
| Chemical data | |
| Formula | C27H36N2O5 |
| Molecular mass | 468.585 g/mol
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
  COCK SAYS MOM CAN TEACH YOU NMR COCK SAYS MOM CAN TEACH YOU NMR

|




No comments:
Post a Comment