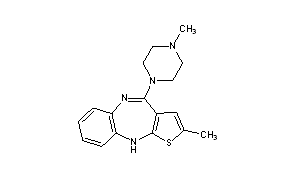

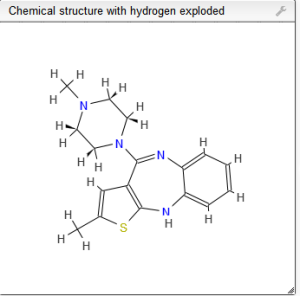
Olanzapine, LY170053
CAS : 132539-06-1
2-Methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine
2-Methyl-4-(4-methyl-1-piperazinyl)-10Hthieno[2,3-b][1,5]benzodiazepine
Manufacturers' Codes: LY-170053
Trademarks: Zyprexa (Lilly)
Molecular Formula: C17H20N4S
Molecular Weight: 312.43
Percent Composition: C 65.35%, H 6.45%, N 17.93%, S 10.26%
Properties: Crystals from acetonitrile, mp 195°. Practically insol in water.
Melting point: mp 195°
Therap-Cat: Antipsychotic.
Keywords: Antipsychotic; Other Tricyclics; Serotonin-Dopamine Antagonist.

Olanzapine (sold under the brand names Zyprexa, Zypadhera and Lanzek or in combination with fluoxetine, Symbyax) is anatypical antipsychotic. It is approved by the U.S. Food and Drug Administration (FDA) for the treatment of schizophrenia and bipolar disorder.[4]
Olanzapine is structurally similar to clozapine and quetiapine, but is classified as a thienobenzodiazepine. The olanzapine formulations are manufactured and marketed by the pharmaceutical company Eli Lilly and Company; the drug went generic in 2011. Sales of Zyprexa in 2008 were $2.2B in the US, and $4.7B worldwide.[5]
Zyprexa (olanzapine) 10 mg tablets (AU)
Olanzapine has a higher affinity for 5-HT2A serotonin receptors than D2 dopamine receptors, which is a common property of all atypical antipsychotics, aside from the benzamide antipsychotics such as amisulpride. Olanzapine also had the highest affinity of any second-generation antipsychotic towards the P-glycoprotein in one in vitro study.[60] P-glycoprotein transports a number of drugs across a number of different biological membranes including the blood-brain barrier, which could mean that less brain exposure to olanzapine results from this interaction with the P-glycoprotein.[61]
Olanzapine is a potent antagonist of the muscarinic M3 receptor,[65] which may underlie its diabetogenic side effects.[64][66] Additionally, olanzapine also exhibits a relatively low affinity for serotonin 5-HT1, GABAA, beta-adrenergic receptors, and benzodiazepine binding sites.[67] [27]
Dosage forms
Olanzapine is marketed in a number of countries, with tablets ranging from 2.5 to 20 milligrams. Zyprexa (and generic olanzapine) is available as an orally-disintegrating "wafer" which rapidly dissolves in saliva. It is also available in 10 milligram vials for intramuscular injection.[4]
Research
Olanzapine has been investigated for use as an antiemetic, particularly for the control of chemotherapy-induced nausea and vomiting (CINV). A 2007 study demonstrated its successful potential for this use, achieving a complete response in the acute prevention of nausea and vomiting in 100% of patients treated with moderately and highly-emetogenic chemotherapy, when used in combination with palonosetron and dexamethasone.[85]
Olanzapine has been considered as part of an early psychosis approach for schizophrenia. The Prevention through Risk Identification, Management, and Education (PRIME) study, funded by the National Institute of Mental Health and Eli Lilly, tested the hypothesis that olanzapine might prevent the onset of psychosis in people at very high risk forschizophrenia. The study examined 60 patients with prodromalschizophrenia, who were at an estimated risk of 36–54% of developing schizophrenia within a year, and treated half with olanzapine and half with placebo.[86] In this study, patients receiving olanzapine did not have a significantly lower risk of progressing to psychosis. Olanzapine was effective for treating the prodromal symptoms, but was associated with significant weight gain.[87]
1H NMR PREDICT

13C NMR PREDICT

COSY
HMBC
.........................................WILL BE UPDATED
UV
IR
1H NMR
13C NMR
MASS
...........................................
INTERMEDIATE/S USED IN SYNTHESIS AND REFERENCE


LEK PHARMACEUTICALS D.D. Patent: WO2005/90359 A2, 2005 ; Location in patent: Page/Page column 21 ;


Apotex Pharmachem Inc. Patent: US2008/319189 A1, 2008 ; Location in patent: Page/Page column 2 ;


LEK PHARMACEUTICALS D.D. Patent: WO2005/90359 A2, 2005 ; Location in patent: Page/Page column 21 ;


Leyva-Perez, Antonio; Cabrero-Antonino, Jose R.; Corma, Avelino Tetrahedron, 2010 , vol. 66, # 41 p. 8203 - 8209
SEE
WATSON PHARMACEUTICALS, INC. Patent: WO2004/94390 A1, 2004 ; Location in patent: Page 15 ;

WO2006/6180 A1, ; Page/Page column 11 ;
 Russian Journal of Bioorganic Chemistry, , vol. 31, # 4 p. 378 - 382
Russian Journal of Bioorganic Chemistry, , vol. 31, # 4 p. 378 - 382 Russian Journal of Bioorganic Chemistry, , vol. 31, # 4 p. 378 - 382
Russian Journal of Bioorganic Chemistry, , vol. 31, # 4 p. 378 - 382 WO2006/6180 A1, ; Page/Page column 11 ;
WO2006/6180 A1, ; Page/Page column 11 ; US5605897 A1, ;
US5605897 A1, ;
.............................................
PATENT


Figure 2 shows the NMR spectrum of the solvate according to the invention. The peaks were assigned as follows (1H NMR; CDCl3, 300 MHz) :
| Chemical shift δ | Assignement |
| 1.20 (3H, d) | CH3 - isopropanol |
| 2.30 (3H, s) | 4'-CH3 |
| 2.34 (3H, s) | 2- CH3 |
| 2.20-2.40 (2H, br s) | H - water |
| 2.49 (4H, m) | 3'-CH2 |
| 3.52 (4H, m) | 2'-CH2 |
| 4.03 (0.5H, dq) | CH - isopropanol |
| 5.02 (H, broad s) | 10-NH |
| 6.29 (H, broad s) | 3-CH |
| 6.29-7.05 (4H, m) | 6, 7, 8, 9-H |
- Olanzapine has shown to have high activity with regard to the central nervous system and is also useful for the treatment of schizophrenia, schizophreniform disorders, acute mania, mild anxiety states and psychosis.
- Various polymorphic and pseudopolymorphic forms, such as solvates, of olanzapine have become known. Some of them are useful for conversion to other desirable forms.
- The British patent GB 1 533 235 discloses antipsychotically effective thienobenzodiazepines by a generic formula which also covers olanzapine.
- US patent 5,229,382 discloses olanzapine explicitly. The described process for its synthesis involves a crystallization from acetonitrile.
- EP-B-733 635 claims crystalline form II olanzapine, and this polymorphic form is said to be more stable than the material obtained according to US 5,229,382 which is designated "form I olanzapine". Both the form I and the form II of olanzapine are characterized by e. g. X-ray data. The preparation of the more stable form II of olanzapine is effected by dissolving technical grade olanzapine in ethyl acetate and crystallization from the resulting solution by any conventional process such as seeding, cooling, scratching the glass of the reaction vessel or other common techniques.
- WO 02/18390 discloses the monohydrate form I and the dihydrate form I of olanzapine, a process for production thereof and a process for production of form I of olanzapine which comprises the steps of stirring olanzapine monohydrate form I or crude olanzapine or form II of olanzapine in methylene chloride at reflux, cooling, filtering and drying. It is also described that a repeating of the process described in US 5,229,382 Example 1, subexample 4 did not lead to formation of form I of olanzapine.
- WO 03/101997 relates to processes for preparation of form I of olanzapine by regulation of the pH-value of the solution.
- WO 03/055438 discloses the preparation of form I olanzapine by crystallization from ethanol and subsequent conversion of the obtained ethanol solvate.
- US patent 5,637,584 discloses the (mono)methylene chloride solvate form of olanzapine and a method for its conversion to the polymorphic form I of olanzapine.
- EP-B-733 634 discloses three specific solvates of olanzapine, namely the methanol, ethanol and 1-propanol solvates and a process for production of form II olanzapine by drying such a solvate.
- WO 03/097650 describes two new, mixed solvate forms, the water/methylene chloride solvate and the water/DMSO solvate, methods for preparing them, and their transformation to polymorphic form I.
- WO 2004/006933 discloses a process for the preparation of form I olanzapine, as well as various pseudopolymorphic forms, namely the isopropanol solvate, and the acetonitrile/methylene chloride/water and acetonitrile/water mixed solvates of olanzapine, and the polymorphic form A.

Preparation of the water-isopropanol mixed solvate of olanzapine Example 1
- A mixture of 4-amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine hydrochloride (26.6 g), 1-methylpiperazine (92 ml), dimethylsulfoxide (120 ml) and toluene (120 ml) was refluxed for 4 hours. The solution was cooled to 95°C and 200 ml were distilled off under vacuum. The residue was cooled to room temperature, isopropanol (180 ml) was added, and the solution was further cooled to 0°C and water (36 ml) was added to initialize crystallization. After the crystallization was completed, the precipitate was filtered off and washed with isopropanol (20 ml). The wet product was suspended in isopropanol (200 ml) and heated to reflux to obtain a clear solution. Ethylenediaminotetraacetic acid disodium salt (3 g) was added and the suspension was stirred for one hour. Undissolved material was removed by hot filtration. The clear solution was cooled to 25°C and water (6 ml) was added to start crystallization. The suspension was cooled to 0°C and after completion of the crystallization the product was filtered off and washed with isopropanol (10 ml). The product was dried at room temperature under vacuum to a constant weight. Yield: 22.84 g. Loss on drying (140°C): 13.6%. Water content (Karl Fischer): 5.12%.
Example 2
- A mixture of 4-amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine hydrochloride (26.6 g), 1-methylpiperazine (92 ml), dimethylsulfoxide (36 ml) and toluene (120 ml) was refluxed for 4 hours. The solution was cooled to 95°C and 80 ml were distilled off under vacuum. The residue was cooled to room temperature, and isopropanol (180 ml) was added. The solution was further cooled to 0°C and water (36 ml) was added to initialize crystallization. After the crystallization was completed, the precipitate was filtered off and washed with isopropanol (20 ml). The wet product was suspended in isopropanol (200 ml) and heated to reflux to obtain a clear solution. Ethylenediaminotetraacetic acid disodium salt (3 g) was added and the suspension was stirred for one hour. Undissolved material was removed by hot filtration. The clear solution was cooled to 35 °C and water (6 ml) was added to start crystallization. The suspension was cooled to 0°C, upon finalization of the crystallization, the product was filtered off and washed with isopropanol (10 ml). The product was dried at room temperature under vacuum to a constant weight. Yield: 21.98 g. Loss on drying (140°C): 13.2 %. Water content (Karl Fischer): 5.09%. Assay of isopropanol (GC): 8.55 %.
Example 3
- A mixture of 4-amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine hydrochloride (26.6 g), 1-methylpiperazine (92 ml), dimethylsulfoxide (36 ml) and toluene (120 ml) was refluxed for 4 hours. The solution was cooled to 95°C and 120 ml were distilled off under vacuum. The residue was cooled to room temperature, and isopropanol (180 ml) was added. The solution was further cooled to 0°C and water (36 ml) was added to initialize crystallization. After completion of the crystallization, the precipitate.was filtered off and washed with isopropanol (20 ml). The wet product was suspended in isopropanol (200 ml) and heated to reflux to obtain a clear solution. Ethylenediaminotetraacetic acid disodium salt (3 g) was added and the suspension was stirred for one hour. Undissolved material was removed by hot filtration. The clear solution was cooled to 35°C and water (6 ml) was added to start crystallization. The suspension was cooled to 0°C, upon completion of the crystallization, the product was filtered off and washed with isopropanol (10 ml). The product was dried at room temperature under vacuum to a constant weight. Yield: 24.35 g. Loss on drying (140°C): 13.5%. Water content (Karl Fischer): 5.05%.
Example 4
- Anhydrous olanzapine (10 g) was suspended in isopropanol (108 ml) and heated to reflux to obtain a clear solution. The solution was slowly cooled. Water (6 ml) was added at 57°C to start crystallization. The suspension was cooled to 0°C, upon finalization of the crystallization, the product was filtered off and washed with isopropanol (5 ml). The product was dried at room temperature under vacuum to a constant weight. Yield: 10.97 g. Loss on drying (140°C): 13.3%. Water content (Karl Fischer): 5.13%.
Example 5
- 60 g of olanzapine obtained from mother liquors was suspended in isopropanol (650 ml) and heated to reflux to obtain a clear solution. Ethylenediaminotetraacetic acid disodium salt (7.9 g) was added and the suspension was stirred for one hour. Undissolved material was removed by hot filtration. The clear solution was cooled to 25°C and water (16 ml) was added to start crystallization. The suspension was cooled to 0°C and, upon completion of the crystallization, the product was filtered off and washed with isopropanol (50 ml). The product was dried at room temperature under vacuum to a constant weight. Yield: 57.64 g. Loss on drying (140°C): 13.5%. Water content (Karl Fischer): 5.26%.
Example 6
- The solution of 2,4-bis(4-methyl-1-piperazinyl)-3-propylidene-3H-[1,5]benzodiazepine (41.86 g, 0.11 mmol) (prepared according to WO 2004/065390 ), pyridinium p-toluenesulfonate (55.29 g, 0.22 mmol) and sulfur (11.99 g, 0.374 mmol) in benzonitrile (1100 mL) was stirred at 140°C for 11 h, cooled to 90°C and concentrated to an oily residue. The residue was diluted with dichloromethane and isopropanol (250 mL, 1 : 1). The precipitate was filtered off and washed with dichloromethane and isopropanol (20 ml, 1 : 1). The filtrate was extracted with HCl (250 ml, 2 M). The organic phase was further extracted with HCl (2 X 100 ml, 1 M). The combined aqueous phases were cooled in an ice bath and made alkaline by using 5 M NaOH. The obtained turbid solution was left in a refrigerator over night resulting in a suspension. This was separated by filtration and washed with isopropanol (2 X 25 ml). The wet material was suspended in isopropanol (215 ml) and heated to reflux to obtain a clear solution. The solution was hot filtered. Water (6.5 ml) was added to induce crystallization. The obtained'suspension was cooled to 0°C, and upon completion of crystallisation, the product was filtered off and washed with isopropanol (10 ml). The product was dried at room temperature under vacuum to a constant weight. Yield: 18.61 g. Loss on drying (140°C): 12.8 %. Water content (Karl Fischer): 5.29 %.
Example 7
- The solution of 2,4-bis(4-methyl-1-piperazinyl)-3-propylidene-3H-[1,5]benzodiazepine (3.805 g, 10 mmol) (prepared according to WO 2004/065390 ), pyridinium p-toluenesulfonate (5.026 g, 20 mmol) and sulfur (1.122 g, 35 mmol) in benzonitrile (100 ml.) was stirred at 140°C for 8.5 h, cooled to 90°C and concentrated to an oily residue. The residue was diluted with isopropanol (50 ml) and dimethyl sulfoxide (5 ml). The precipitate was filtered off and washed with isopropanol (5 ml). Water (10 ml) and sodium hydroxide (1.00 g, 25 mmol) were added to the filtrate. The mixture was stirred at room temperature until the sodium hydroxide had dissolved. The turbid solution was left in a refrigerator over night resulting in a suspension. This was filtered off and washed with isopropanol (5 mL). The wet material was suspended in isopropanol (25 mL) and the suspension was heated to reflux. Then solids were hot filtered. Water (0.75 mL) was added to the filtrate to induce crystallization. The resulting suspension was cooled to 0°C, and upon completion of crystallisation, the product was filtered off and washed with isopropanol (1 mL). The product was then dried at room temperature under vacuum to a constant weight. Yield: 0.738 g.
......................................
PAPER

Scheme 1
Synthesis of compounds 8a, 8b, and 8c. Reagents and conditions: (i) 1-fluoro-2-nitrobenzene, NaH, THF, rt, 20 h, 60%; (ii) SnCl2, EtOH, 80°C, 1 h, 80%; (iiia) N-methylhomopiperazine (5 equiv), no solvent, microwave heating, 80°C, 4 h,65%; (iiib) N-methylhomopiperazine (5 equiv), no solvent, microwave heating, 120°C, 3 h, 55%; (iiic) N-methylpiperazine (10 equiv), N-methylpiperazine hydrochloride (10 equiv), DMSO, 110–120°C, 20 h, 56%.
References
- Burton, Michael E.; Shaw, Leslie M.; Schentag, Jerome J.; Evans, William E. (May 1, 2005). Applied Pharmacokinetics & Pharmacodynamics: Principles of Therapeutic Drug Monitoring (4th ed.). Lippincott Williams & Wilkins. p. 815. ISBN 978-0-7817-4431-7.
- "PRODUCT INFORMATION OLANZAPINE SANDOZ® 2.5mg/5mg/7.5mg/10mg/15mg/20mg FILM-COATED TABLETS" (PDF). TGA eBusiness Services. Sandoz Pty Ltd. 8 June 2012. Retrieved 26 November 2013.
- "Zyprexa, Zyprexa Relprevv (olanzapine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 26 November 2013.
- ^ Jump up to:a b c "Olanzapine Prescribing Information" (PDF). Eli Lilly and Company. 2009-03-19. Retrieved 2009-09-06.
- "Lilly 2008 Annual Report" (PDF). Lilly. 2009. Retrieved 2009-08-06.
- National Collaborating Centre for Mental Health (25 March 2009). "Schizophrenia: Full national clinical guideline on core interventions in primary and secondary care". Retrieved 25 November 2009.
- Duggan L, Fenton M, Dardennes RM, El-Dosoky A, Indran S (2005). Duggan, Lorna, ed. "Olanzapine for schizophrenia". Cochrane Database of Systematic Reviews (2): CD001359.doi:10.1002/14651858.CD001359.pub2. PMID 10796640.
- "Psychosis and schizophrenia in adults: treatment and management | Guidance and guidelines | NICE". National Institute for Health and Care Excellence.
- Barnes TR (2011). "Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology". J. Psychopharmacol. (Oxford) 25 (5): 567–620. doi:10.1177/0269881110391123.PMID 21292923.
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Möller HJ (2013). "World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects". World J. Biol. Psychiatry 14 (1): 2–44. doi:10.3109/15622975.2012.739708. PMID 23216388.
- Abou-Setta, AM; Mousavi, SS; Spooner, C; Schouten, JR; Pasichnyk, D; Armijo-Olivo, S; Beaith, A; Seida, JC; Dursun, S; Newton, AS; Hartling, L (August 2012).PMID 23035275. Missing or empty
|title=(help) - Zhang JP, Gallego JA, Robinson DG, Malhotra AK, Kane JM, Correll CU (July 2013)."Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis". Int. J. Neuropsychopharmacol. 16 (6): 1205–18. doi:10.1017/S1461145712001277.PMC 3594563. PMID 23199972.
- Citrome L (August 2012). "A systematic review of meta-analyses of the efficacy of oral atypical antipsychotics for the treatment of adult patients with schizophrenia". Expert Opin Pharmacother 13 (11): 1545–73. doi:10.1517/14656566.2011.626769.PMID 21999805.
- Lepping P, Sambhi RS, Whittington R, Lane S, Poole R (May 2011). "Clinical relevance of findings in trials of antipsychotics: systematic review". Br J Psychiatry 198 (5): 341–5.doi:10.1192/bjp.bp.109.075366. PMID 21525517.
- Désaméricq G, Schurhoff F, Meary A et al. (February 2014). "Long-term neurocognitive effects of antipsychotics in schizophrenia: a network meta-analysis". Eur. J. Clin. Pharmacol. 70 (2): 127–34. doi:10.1007/s00228-013-1600-y. PMID 24145817.
- Leucht S, Cipriani A, Spineli L et al. (September 2013). "Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis".Lancet 382 (9896): 951–62. doi:10.1016/S0140-6736(13)60733-3. PMID 23810019.
- Osser DN, Roudsari MJ, Manschreck T (2013). "The psychopharmacology algorithm project at the Harvard South Shore Program: an update on schizophrenia". Harv Rev Psychiatry 21 (1): 18–40. doi:10.1097/HRP.0b013e31827fd915. PMID 23656760.
- [+http://www.nice.org.uk/guidance/cg185/chapter/1-recommendations "Bipolar disorder: the assessment and management of bipolar disorder in adults, children and young people in primary and secondary care | 1-recommendations | Guidance and guidelines | NICE"].
- Yatham LN, Kennedy SH, O'Donovan C et al. (December 2006). "Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007". Bipolar Disord 8 (6): 721–39. doi:10.1111/j.1399-5618.2006.00432.x. PMID 17156158.
- Selle V, Schalkwijk S, Vázquez GH, Baldessarini RJ (March 2014). "Treatments for acute bipolar depression: meta-analyses of placebo-controlled, monotherapy trials of anticonvulsants, lithium and antipsychotics". Pharmacopsychiatry 47 (2): 43–52.doi:10.1055/s-0033-1363258. PMID 24549862.
- Maglione M, Maher AR, Hu J et al. (2011). "Off-Label Use of Atypical Antipsychotics: An Update". PMID 22132426.
- Review of olanzapine in the management of bipolar disorders Neuropsychiatr Dis Treat. 2007 October; 3(5): 579–587.
- Scott, Lisa (Winter 2006). "Genetic and Neurological Factors in Stuttering". Stuttering Foundation of America.
- "Important Safety Information for Olanzapine". Zyprexa package insert. Eli Lilly & Company. 2007. Archived from the original on 2007-11-23. Retrieved 2007-12-03.
Elderly patients with dementia-related psychosis treated with atypical antipsychotic drugs are at an increased risk of death compared to placebo. [...] ZYPREXA (olanzapine) is not approved for the treatment of elderly patients with dementia-related psychosis.
- "Doctors 'ignoring drugs warning'". BBC News. 17 June 2008. Retrieved 2008-06-22.
- L-Z\In re Zyprexa\Documents Leak\Injunction Memo & Order\FINAL INJUNCTION MEMO 2.13.07.wpd
- EFF.org
- Press Releases: January, 2007 | Electronic Frontier Foundation
- Eli Lilly was Concerned by Zyprexa Side-Effects from 1998, The Times (London), January 23, 2007
- Navari RM, Einhorn LH, Loehrer PJ, Passik SD, Vinson J, McClean J, Chowhan N, Hanna NH, Johnson CS (2007). "A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: A Hoosier oncology group study". Supportive Care in Cancer 15 (11): 1285–91. doi:10.1007/s00520-007-0248-5. PMID 17375339.
- McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller TJ, Woods SW, Hawkins KA, Hoffman R, Lindborg S, Tohen M, Breier A (2003). "The PRIME North America randomized double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis". Schizophrenia Research 61 (1): 7–18.doi:10.1016/S0920-9964(02)00439-5. PMID 12648731.
- McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller T, Woods SW, Hawkins KA, Hoffman RE, Preda A, Epstein I, Addington D, Lindborg S, Trzaskoma Q, Tohen M, Breier A (2006). "Randomized, Double-Blind Trial of Olanzapine Versus Placebo in Patients Prodromally Symptomatic for Psychosis". American Journal of Psychiatry 163 (5): 790–9.
Literature References:
Serotonin (5-HT2) and dopamine (D1/D2) receptor antagonist with anticholinergic activity. Prepn: J. K. Chakrabarti et al., EP 454436; eidem, US 5229382 (1991, 1993 both to Lilly). PRODUCT PATENT
Comparative pharmacology: N. A. Moore et al., Curr. Opin. Invest. Drugs 2, 281 (1993). HPLC determn in human plasma: J. T. Catlow et al., J. Chromatogr. B 668, 85 (1995).
Clinical evaluation in schizophrenia: D. S. Baldwin, S. A. Montgomery, Int. Clin. Psychopharmacol. 10, 239 (1995); in mania of bipolar disorder: M. Tohen et al., Am. J. Psychiatry 156, 702 (1999).
Review of pharmacology and clinical experience: B. C. Lund, P. J. Perry, Expert Opin. Pharmacother. 1, 305-323 (2000).
External links
- Zyprexa.com - official Zyprexa brand website from Eli Lilly and Company
- Zyprexa package insert
- Berenson, Alex (December 17, 2006). "Lilly Settles With 18,000 Over Zyprexa". New York Times.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.

 COCK WILL TEACH YOU NMR
COCK WILL TEACH YOU NMR COCK SAYS MOM CAN TEACH YOU NMR
COCK SAYS MOM CAN TEACH YOU NMR
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE


No comments:
Post a Comment