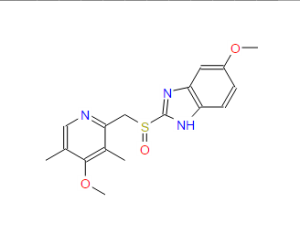
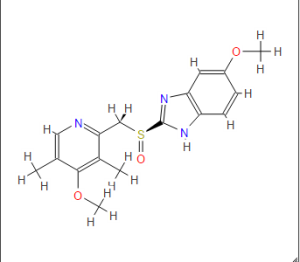
Omeprazole
CAS NO. 119141-89-8
(RS)-5-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl) methylsulfinyl)-1H-benzo[d]imidazole
| Omeprazole | |
| CAS NO.: | 119141-89-8 |
|---|---|
| SYNONYMS: |
|
| FORMULA: | C17H19N3O3S |
| EXACT MASS: | 345.11500 |
Ome is a chemical substance (C17H19N3O3S), its molecular weight is 345.42g/mol, the color is white, has weak alkaline properties, melts at 156oC
NMR...............http://file.selleckchem.com/downloads/nmr/S138902-Omeprazole-Prilosec-HNMR-Selleck.pdf
NMR...........file:///C:/Users/anthonyc/Downloads/233-434-1-SM.pdf

1H NMR PREDICT

13C NMR PREDICT

COSY
HMBC

......................................
ARKIVOC 2006 (v) 5-11
The structure of Omeprazole in the solid state: a 13C and 15N NMR/CPMAS study
Rosa M. Claramunt,a Concepción López,a and José Elguerob *
a Departamento de Química Orgánica y Bio-Orgánica, Facultad de Ciencias, UNED, Senda del Rey 9, E-28040 Madrid, Spain
b Instituto de Química Médica, CSIC, Juan de la Cierva, 3. E-28006 Madrid, Spain
E-mail: iqmbe17@iqm.csic.es
Abstract
The 13C and 15N CPMAS spectra of a solid sample of Omeprazole have been recorded and all the signals assigned. The sample consists uniquely of the 6-methoxy tautomer. For analytical purposes, the signals of the other tautomer, the 5-methoxy one, were estimated from the data in solution (Magn. Reson. Chem. 2004, 42, 712).
Keywords: Omeprazole, NMR, 13C, 15N, CPMAS, tautomerism, benzimidazole
Omeprazole, 5(6)-methoxy-2-{(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl}-1H-benz - imidazole [1(2)], is an important ulcer drug,1 that has been classified amongst the blockbuster drugs.2 This compound presents two sources of structural differentiation. First, Omeprazole is chiral (a vs. b) 3 since it has a stereogenic center on the sulfur atom but the commercial form has been sold, until recently, as a racemate. In 2001, Esomeprazole magnesium, the S enantiomer was approved.4 The second source of diversity is that these compounds present tautomerism (1 vs. 2). We have already devoted a paper to the tautomerism of Omeprazole in solution using 1 H and 13C NMR spectroscopy.5 In this paper a complete assignment of the signals was carried out and the tautomeric equilibrium constant, KT = [2]/[1], was determined in THF at 195 K, to be 0.59 in favor of the 6-methoxy tautomer 2.
References
1. Carlsson, E.; Lindberg, P.; von Unge, S. Chem. Brit. 2002, 38, 42 and references therein.
2. Berkowitz, B. A.; Sachs, G. Mol. Interventions 2002, 2, 6.
3. von Unge, S.; Langer, V.; Sjölin, L. Tetrahedron: Asymmetry 1997, 8, 1967.
4. Olbe, L.; Carlsson, E.; Lindberg, P. Nature Reviews Drug Discovery 2003, 2, 132.
5. Claramunt, R. M.; López, C.; Alkorta, I.; Elguero, J.; Yang, R.; Schulman, S. Magn. Reson. Chem. 2004, 42, 712.
6. Elguero, J.; Katritzky, A. R.; Denisko, O. Adv. Heterocycl. Chem. 2000, 76. 1.
7. Allen, F. H. Acta Crystallogr. Sect. B 2002, 58, 380.
8. Braga, S. S.; Ribeiro-Claro, P.; Pillinger, M.; Gonçalves, I. S.; Fernandes, A. C.; Pereira, F.; Romåo, C. C.; Correia, P. B.; Teixeira-Dias, J. J. C. J. Incl. Phenom. Macro. Chem. 2003, 47, 47.
9. Berger, S.; Braun, S. 200 and More NMR Experiments. Wiley-VCH, Weinheim, 2004.
DSC OF OMEPRAZOLE
UV
UV Study: The Ultraviolet spectrum was recorded from 200 nm to 400 nm, with API concentration of 0.0015% in methanol. The spectrum showed two λmax at 207 and 301 nm. As seen below.
FTIR Study The FTIR of spectrum of Omeprazole was recorded by preparation of pellet with KBr.
NMR
13 C NMR
mass
The mass spectrum of Omeprazole was recorded on 4000-Q trap LCMSMS system. The sample is introduced into the system through HPLC by bypassing the column. The ESI +ve ionization spectrum of Omeprazole displayed a protonated molecular ion at m/z= 346 which corresponds to the molecular formula C17H17N3O3S. The fragmentation pattern was observed with product ion scan.
Raman
Title: Omeprazole
CAS Registry Number: 73590-58-6
CAS Name: 5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole
Manufacturers' Codes: H-168/68
Trademarks: Gastrogard (Merial); Losec (AstraZeneca); Mopral (AstraZeneca); OmeLich (Winthrop); Omelind (Lindopharm); Omepral (AstraZeneca); Omeprazen (Malesci); Osiren (Probiomed); Parizac (Lacer); Pepticum (Grñenthal); Prilosec (AstraZeneca); Zegerid (Santarus); Zoltum (AstraZeneca)
Molecular Formula: C17H19N3O3S
Molecular Weight: 345.42
Percent Composition: C 59.11%, H 5.54%, N 12.16%, O 13.90%, S 9.28%
Literature References: Gastric proton-pump inhibitor. Prepn: U. K. Junggren, S. E. Sjostrand, EP 5129; eidem, US 4255431(1979, 1981 both to AB Hässle). Resolution and activity of enantiomers: P. Erlandsson et al., J. Chromatogr. 532, 305 (1990). Manuf process for optically pure salts: S. Von Unge, US 5693818 (1997 to Astra). Pharmacology: P. Muller et al., Arzneim.-Forsch. 33, 1685 (1983). Mechanism of action study: B. Wallmark et al., Biochim. Biophys. Acta 778, 549 (1984). LC determn in plasma and urine: P. Lagerstrom, B. Persson, J. Chromatogr. 309, 347 (1984). Survey of preclinical data: Scand. J. Gastroenterol. 20, Suppl 108, 1-120 (1985). Toxicological studies: L. Ekman et al., ibid. 53. Clinical trial in Zollinger-Ellison syndrome: C. B. H. W. Lamers et al., N. Engl. J. Med. 310, 758 (1984); in duodenal ulcer: K. Lauritsen et al., ibid. 312, 958 (1985). Veterinary trial in race horses: M. J. Murray et al., Equine Vet. J. 29, 425 (1997). Review of pharmacology and clinical efficacy: H. D. Langtry, M. I. Wilde, Drugs 56, 447-486 (1998).
Properties: Crystals from acetonitrile, mp 156°. Freely sol in ethanol, methanol; slightly sol in acetone, isopropanol; very slightly sol in water. LD50 in mice, rats (g/kg): 0.08, >0.05 i.v.; >4, >4 orally (Ekman).
Melting point: mp 156°
Toxicity data: LD50 in mice, rats (g/kg): 0.08, >0.05 i.v.; >4, >4 orally (Ekman)
Derivative Type: Magnesium salt
CAS Registry Number: 95382-33-5
Trademarks: Antra (AstraZeneca); Gastracid (AWD); Gastroloc (AstraZeneca); Omebeta (Betapharm); Omep (Hexal); Ome-Puren (Alpharma)
Molecular Formula: C34H36MgN6O6S2
Molecular Weight: 713.12
Percent Composition: C 57.26%, H 5.09%, Mg 3.41%, N 11.78%, O 13.46%, S 8.99%
Derivative Type: S-Form
CAS Registry Number: 119141-88-7
Additional Names: Esomeprazole; perprazole
Manufacturers' Codes: H-199/18
Literature References: LC-MS determn in plasma: H. Stenhoff et al., J. Chromatogr. B 734, 191 (1999).
Properties: Colorless syrup. [a]D20 -155° (c = 0.5 in chloroform).
Optical Rotation: [a]D20 -155° (c = 0.5 in chloroform)
Derivative Type: S-Form magnesium salt
CAS Registry Number: 161973-10-0
CAS Name: (T-4)-Bis[5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazolato]magnesium
Additional Names: esomeprazole magnesium
Trademarks: Nexium (AstraZeneca)
Literature References: Review of clinical experience in acid disorders: D. A. Johnson, Expert Opin. Pharmacother. 4, 253-264 (2003).
Properties: White powder. [a]D20 -128.2° (c = 1 in methanol).
Optical Rotation: [a]D20 -128.2° (c = 1 in methanol)
Therap-Cat: Antiulcerative; in treatment of Zollinger-Ellison syndrome.
Therap-Cat-Vet: Antiulcerative.
Keywords: Antiulcerative; Gastric Proton Pump Inhibitor.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.

 COCK WILL TEACH YOU NMR
COCK WILL TEACH YOU NMR COCK SAYS MOM CAN TEACH YOU NMR
COCK SAYS MOM CAN TEACH YOU NMR
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE


Thanks for sharing these blog with us...
ReplyDeleteOmeprazole Pellets Manufacturers in India
Omeprazole decreases the measure of corrosive your stomach makes. It's a broadly utilized treatment for heartburn and indigestion and heartburn. It's additionally taken to forestall and treat stomach ulcers. Now and then omeprazole is taken for an uncommon sickness brought about by a growth in the pancreas or gut called Zollinger-Ellison disorder. At Harbor Compounding Pharmacy we work one-on-one with your doctor to formulate a powerful customized solution for your toughest pain problems Pain Management .
ReplyDelete