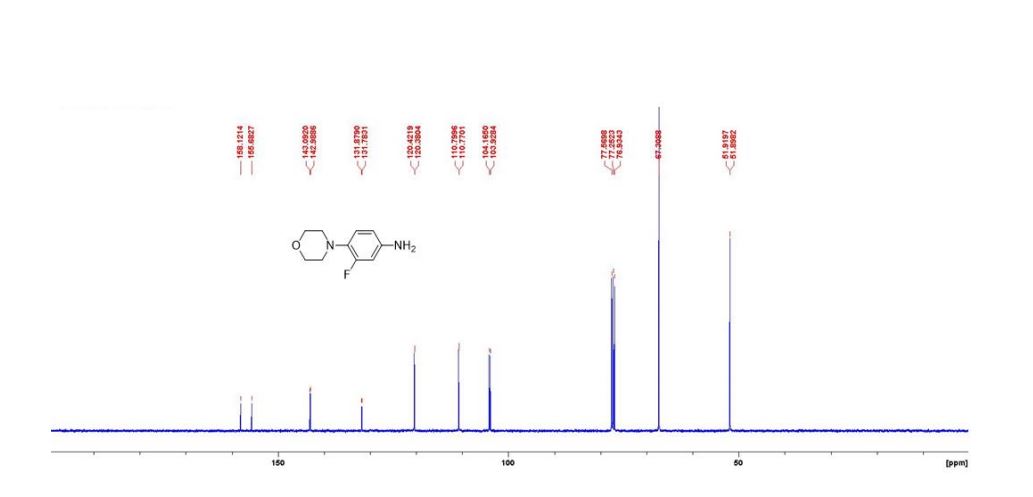
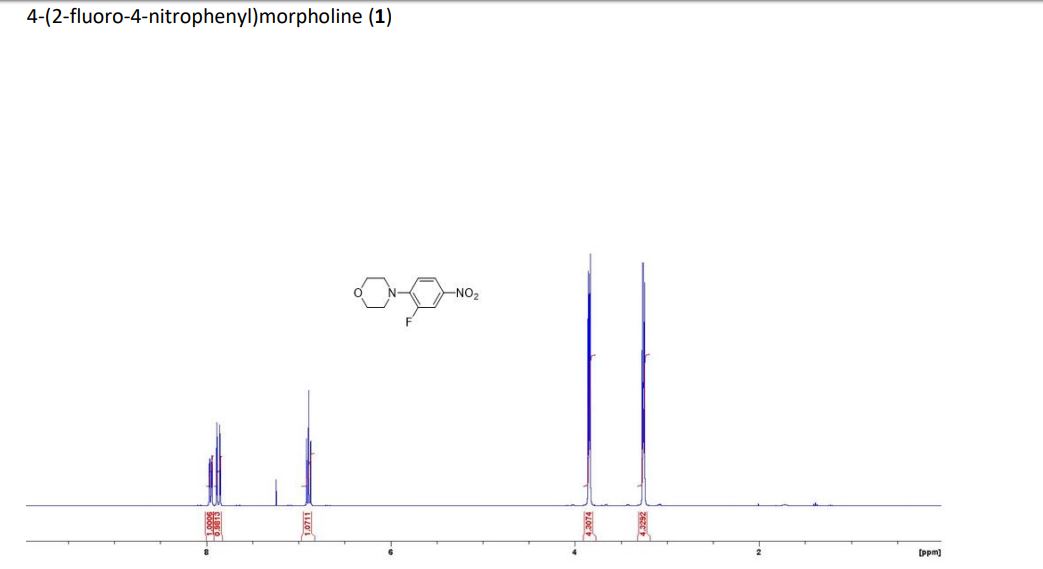
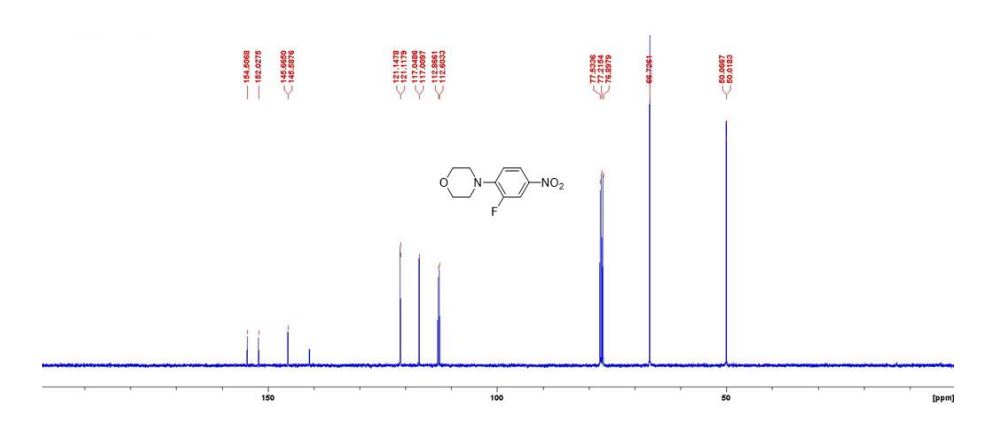
(1S,4R)-2-(4-Methoxybenzoyl)bicyclo[2.2.2]octa-2,5-diene (3a) Yellow liquid (25.2 mg, 95% yield):
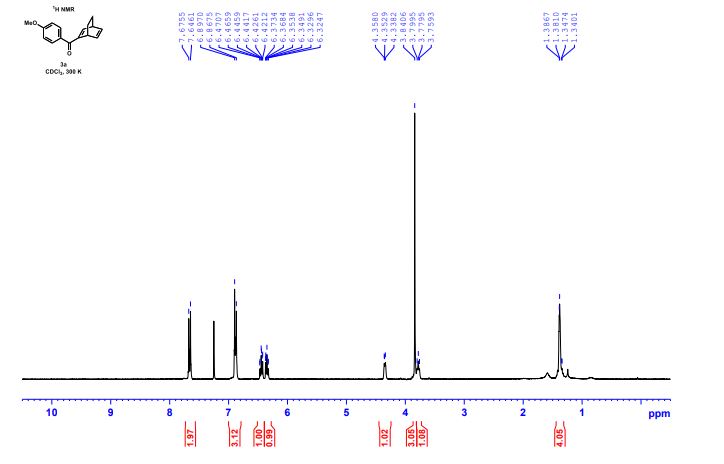
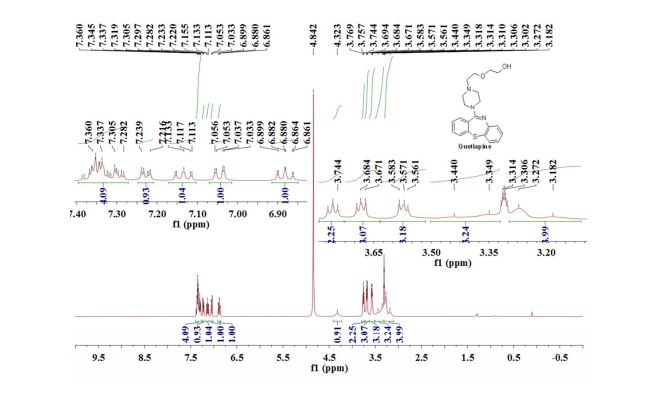
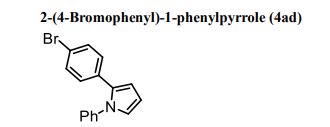
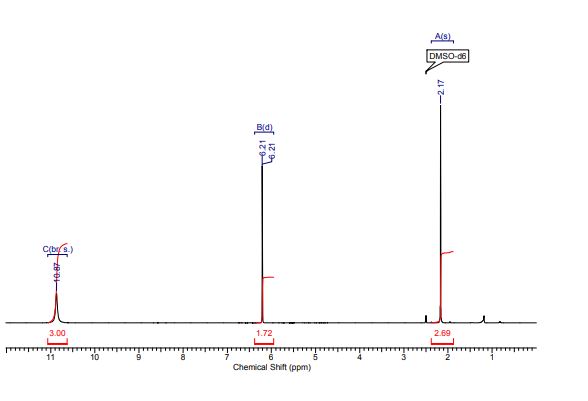
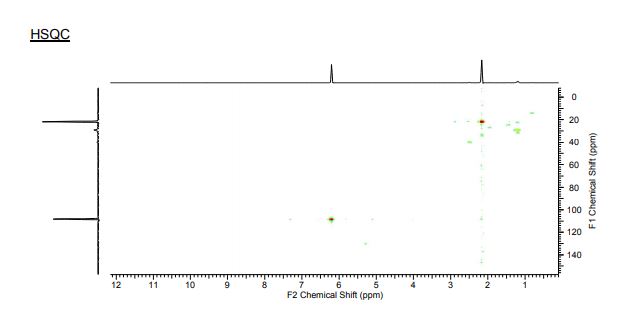
1H NMR (300 MHz, CDCl3) 1.39 (s, 4H, alkyl), 3.77-3.80 (m, 1H, alkyl), 3.85 (s, 3H, OMe), 4.36 (d, J = 5.4 Hz, 1H, alkyl), 6.36 (dd, J = 6.0, 6.0 Hz, 1H, vinyl), 6.46 (dd, J = 6.0, 6.0 Hz, 1H, vinyl), 6.88-6.91 (m, 3H, vinyl + arom.), 7.67 (d, J = 8.3 Hz, 2H, arom.);
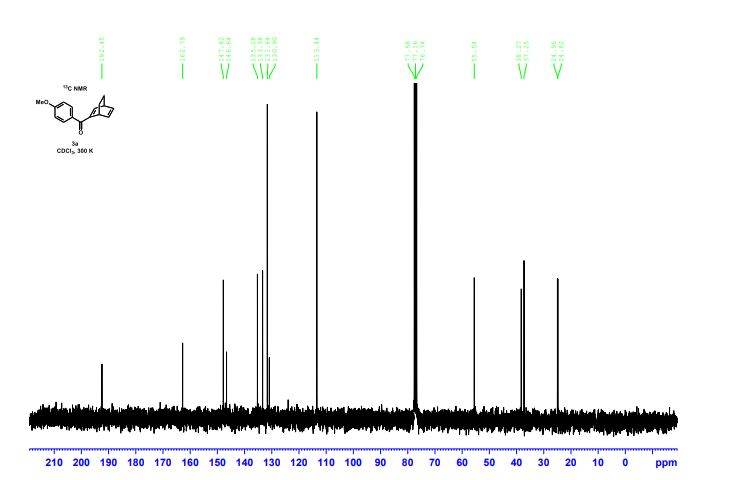
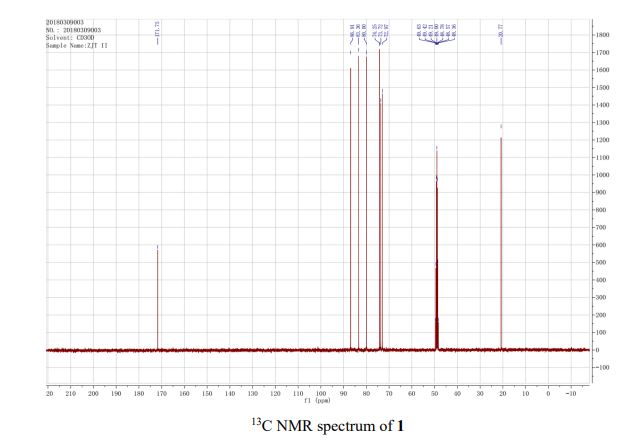
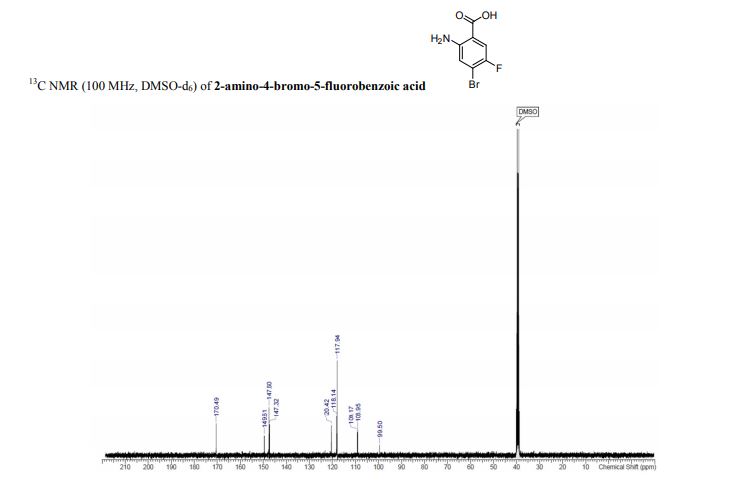
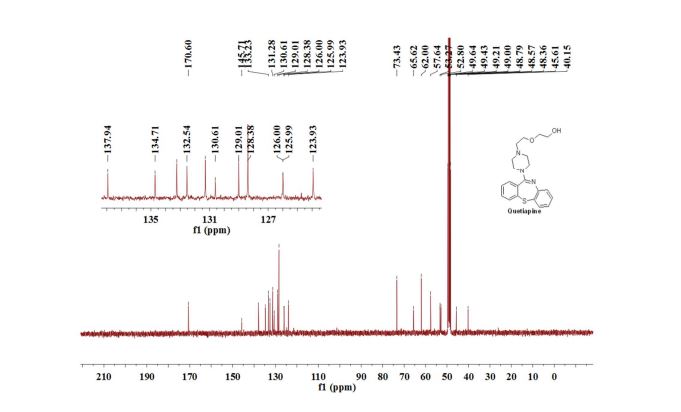
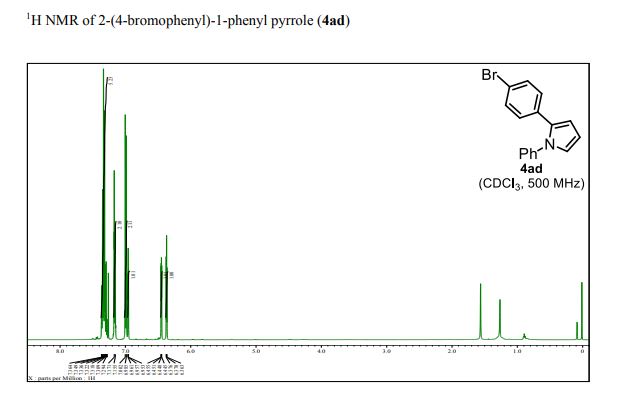
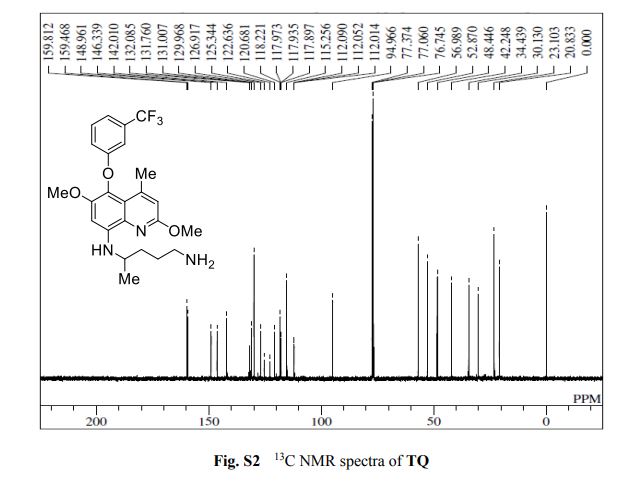
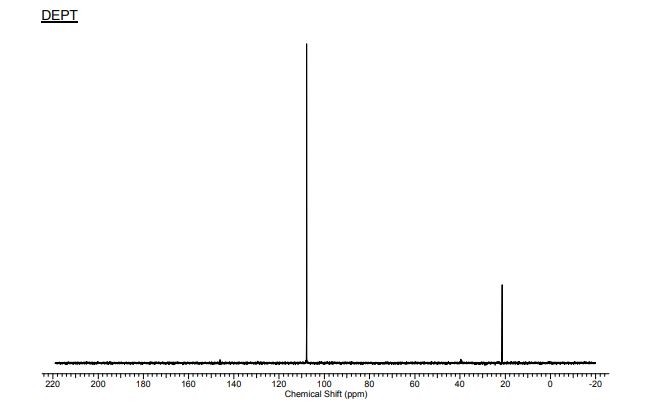
13C{1H} NMR (75 MHz, CDCl3) = 24.7, 24.8, 37.1, 38.2, 55.4, 113.3, 130.8, 131.5, 133.2, 135.1, 146.5, 147.7, 162.6, 192.3;
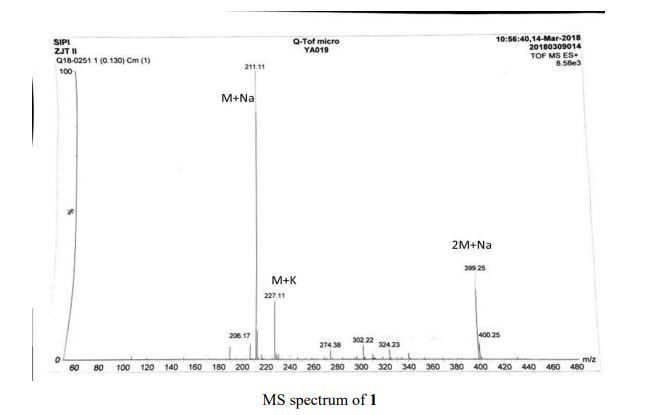
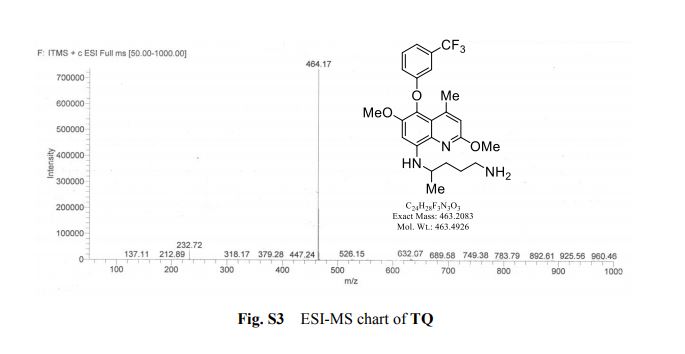
HRMS (ESI-TOF) m/z calculated for C16H16NaO2 [M+Na]+ 263.1048, found 263.1036;
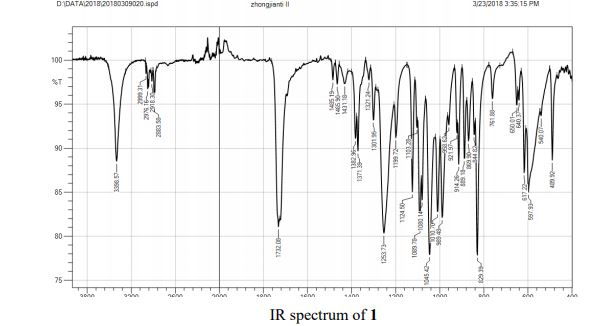
FT-IR (neat, cm-1 ) 1033, 1174, 1255, 1354, 1600, 1637, 1730, 2870, 2957, 3054.
Optical Rotation: []D 26 +39.9 (c 2.52, CHCl3) for an enantiomerically enriched sample of 94% ee.
HPLC analysis (column, CHIRALPAK AD-3, hexane/2-propanol = 98/2, flow rate 1.0 mL/min, 20 C, detection UV 250 nm light); tR of major-isomer 20.7
Org. Lett., 2018, 20 (23), pp 7353–7357
DOI: 10.1021/acs.orglett.8b02263
////////////////
(1S,4R)-2-(4-Methoxybenzoyl)bicyclo[2.2.2]octa-2,5-diene
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
READ
ANTHONY MELVIN CRASTO
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
 amcrasto@gmail.com
amcrasto@gmail.com
CALL +919323115463 INDIA