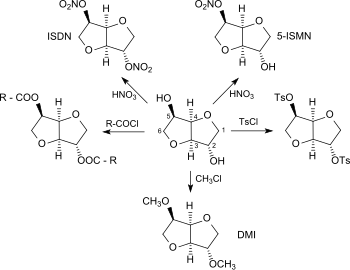
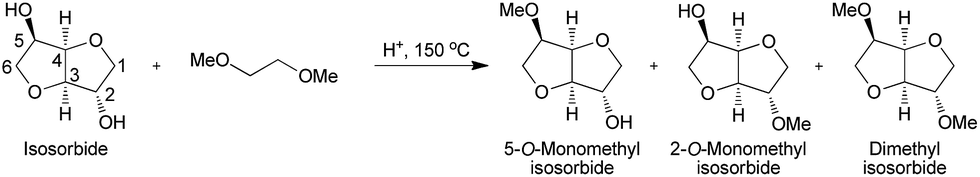
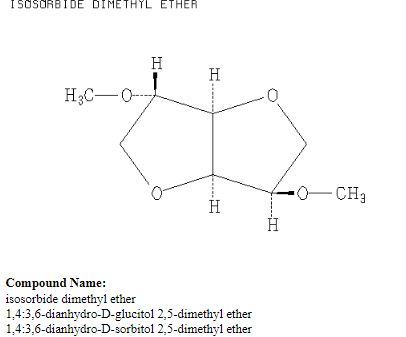
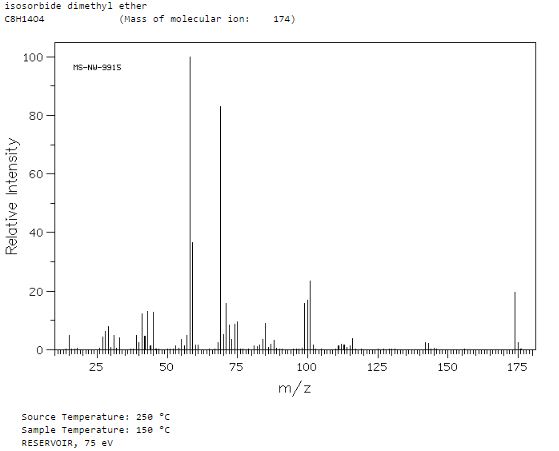
Organic Chemists from Industry and academics to Interact on Spectroscopy Techniques for Organic Compounds ie NMR, MASS, IR, UV Etc. Starters, Learners, advanced, all alike, contains content which is basic or advanced, by Dr Anthony Melvin Crasto, Worlddrugtracker, email me ........... amcrasto@gmail.com, call +91 9323115463 India skype amcrasto64
................DR ANTHONY MELVIN CRASTO Ph.D ( ICT, Mumbai) , INDIA 25Yrs Exp. in the feld of Organic Chemistry,Working for GLENMARK GENERICS at Navi Mumbai, INDIA. Serving chemists around the world. Helping them with websites on Chemistry.Million hits on google, world acclamation from industry, academia, drug authorities for websites, blogs and educational contribution
Pages
- Home
- ABOUT ME
- DIMENSIONS IN NMR SPECTROSCOPY
- 13 C NMR
- 1H NMR
- CHEMDOODLE/INTERACTIVE SPECT PREDICT
- Animations
- HELP ME
- Multinuclear NMR Spectroscopy
- Examples of 13C NMR
- Books on NMR spectroscopy
- UV-Visible Spectroscopy
- IR SPECTRA EXAMPLES
- Journals
- Organic spectroscopy site
- Spectroscopy sites
- IR SPECTROSCOPY
- Books-2
- Recommended Web Sites for Spectra and Spectrum-rel...
- DISCLAIMER
- Mössbauer spectroscopy
- FINDING CHEMICAL SPECTRA
- Mass Spectrometry
- NMR Overview
- Characterisation of Organic Compounds
- SDBS Spectral Database System for Organic Compounds
- CHEMICAL SHIFT
- MASS SPECTROSCOPY
- Books-1
- MASSBANK PORTAL
- 11B NMR
Monday, 16 October 2017
Wednesday, 11 October 2017
Total synthesis of (-)-aritasone via the ultra-high pressure hetero-Diels-Alder dimerisation of (-)-pinocarvone
Total synthesis of (-)-aritasone via the ultra-high pressure hetero-Diels-Alder dimerisation of (-)-pinocarvone
DOI: 10.1039/C7OB02204B, Paper
Maliha Uroos, Phillip Pitt, Laurence M. Harwood, William Lewis, Alexander J. Blake, Christopher J. Hayes
The total synthesis of aritasone via the proposed biosyntheic hetero-Diels-Alder [4 + 2] cyclodimerisation of pinocarvove, has been achieved under ultra-high pressure (19.9 kbar) conditions
The total synthesis of aritasone via the proposed biosyntheic hetero-Diels-Alder [4 + 2] cyclodimerisation of pinocarvove, has been achieved under ultra-high pressure (19.9 kbar) conditions
Total synthesis of (−)-aritasone via the ultra-high pressure hetero-Diels–Alder dimerisation of (−)-pinocarvone
Maliha Uroos,a Phillip Pitt,b Laurence M. Harwood,b William Lewis,a Alexander J. Blakea and Christopher J. Hayes*a
*Corresponding authors
aSchool of Chemistry, University of Nottingham, University Park, Nottingham, UK
E-mail:chris.hayes@nottingham.ac.uk
Fax: +44 (0)115951 3564
Tel: +44 (0)115 951 3045
E-mail:chris.hayes@nottingham.ac.uk
Fax: +44 (0)115951 3564
Tel: +44 (0)115 951 3045
bChemical Sciences Division, University of Reading, Whiteknights, Reading, UK

Christopher Hayes
Abstract
This paper describes a total synthesis of the terpene-derived natural product aritasone via the hetero-Diels–Alder [4 + 2] cyclodimerisation of pinocarvove, which represents the proposed biosyntheic route. The hetero-Diels–Alder dimerisation of pinocarvone did not proceed under standard conditions, and ultra-high pressure (19.9 kbar) was required. As it seems unlikely that these ultra-high pressures are accessible within a plant cell, we suggest that the original biosynthetic hypothesis be reconsidered, and alternatives are discussed.
Aritasone (1) A solution of pinocarvone (()-2) (100 mg, 0.66 mmol) in dichloromethane (5 mL) was pressurized to 19.9 kbar for 120 h. The 1H NMR spectrum of the crude reaction mixture showed significant change in the composition as compared to the starting material. The solvent was evaporated and the residue was purified by column chromatography (pentane/Et2O; 25/1) to afford aritasone (1) (20 mg, 40%) as a white solid; mp 101- 103 C; (lit3 mp 105-106 °C); []D 26 26.1 (c 0.40 in CHCl3); (lit3 []D 9 118); max/cm-1 (CHCl3) 2926, 2359, 1722, 1689, 1601, 1467, 1372, 1305, 1152; H (400 MHz; CDCl3, 298 K) 2.67 (2H, app dd, J 4.8, 2.5, H-2a, H-2b), 2.45-2.32 (3H, m, H-7a, H-15a, H-3), 2.15-2.01 (4H, m, H-10, H-12, H-15b, H-16a), 1.91-1.80 (2H, m, H-4, H-16b), 1.66 (1H, ddd, J 13.8, 6.4, 3.4, H-7b), 1.38 (3H, s, CH3), 1.29-1.22 (7H, br s, CH3, H-13a, H-13b, H-8a, H- 8b), 0.90 (3H, s, CH3), 0.80 (3H, s, CH3); C (100 MHz; CDCl3, 298 K) 209.5 (C), 142.9 (C), 112.8 (C), 80.8 (C), 45.2 (CH), 44.3 (CH), 43.7 (CH2), 40.9 (CH), 40.5 (C), 39.4 (CH), 38.3 (C), 33.2 (CH2), 32.7 (CH2), 27.7 (CH3), 27.3 (CH2), 27.3 (CH3), 26.3 (CH3), 22.5 (CH2), 22.1 (CH2), 20.9 (CH3); HRMS m/z (ES+ ) found 301.2162 (M + H) C20H29O2 requires 301.2162 and 323.1981 (M + Na) C20H28O2Na requires 323.1982. These data were consistent to those previously reported, 5, 7 however the value of the specific rotation5 differs significantly from that measured during the original isolation work.3

Christopher Hayes
Contact
- Room C16 School of Chemistry
University Park
Nottingham
NG7 2RD
UK - 0115 951 3045
- 0115 951 3564
- chris.hayes@nottingham.ac.uk
- http://www.nottingham.ac.uk/~pczcjh/Homepage.html
Biography
Prof. Christopher Hayes began his academic career here in Nottingham with his B.Sc. in July 1992. Remaining at Nottingham, he completed his Ph.D. studies in organic chemistry, under the supervision of Professor Gerald Pattenden, in September 1995. In January 1996, on a NATO Postdoctoral Fellowship, he moved to the University of California at Berkeley where he worked in the group of Professor Clayton H. Heathcock. In September 1997, he returned to Nottingham as a Lecturer in Organic Chemistry, and has subsequently been promoted to Reader (2003), Associate Professor (2006) and Professor of Organic Chemistry (2011).
Research Summary
Research is centred in main-stream synthetic organic chemistry, focusing on the organic chemistry of biologically active molecules. His current research interests span a number of areas such as (i)… read more
Recent Publications
- NICOLLE, SIMON M., LEWIS, WILLIAM, HAYES, CHRISTOPHER J. and MOODY, CHRISTOPHER J., 2016. Stereoselective Synthesis of Functionalized Pyrrolidines by the Diverted N-H Insertion Reaction of Metallocarbenes with -Aminoketone Derivatives: Angewandte Chemie-International Edition Angewandte Chemie-International Edition. 55(11), 3749-3753
- BARTON, NAOMI A., MARSH, BENJAMIN J., LEWIS, WILLIAM, NARRAIDOO, NATHALIE, SEYMOUR, GRAHAM B., FRAY, RUPERT and HAYES, CHRISTOPHER J., 2016. Accessing low-oxidation state taxanes: is taxadiene-4(5)-epoxide on the taxol biosynthetic pathway?: Chemical Science Chemical Science. 7(5), 3102-3107
- PALFRAMAN, MATTHEW J., ALHARTHY, RIMA D., POWALOWSKA, PAULINA K. and HAYES, CHRISTOPHER J., 2016. Synthesis of triazole-linked morpholino oligonucleotides via Cu-I catalysed cycloaddition: Organic & Biomolecular Chemistry Organic & Biomolecular Chemistry. 14(11), 3112-3119
- NICOLLE, SIMON M., HAYES, CHRISTOPHER J. and MOODY, CHRISTOPHER J., 2015. Alkyl Halide-Free Heteroatom Alkylation and Epoxidation Facilitated by a Recyclable Polymer-Supported Oxidant for the In-Flow Preparation of Diazo Compounds: Chemistry-a European Journal Chemistry-a European Journal. 21(12), 4576-4579
Monday, 9 October 2017
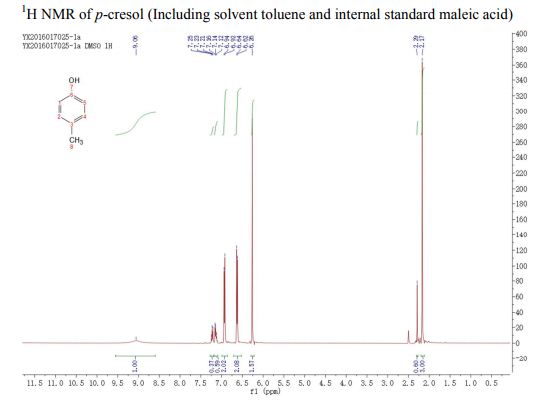
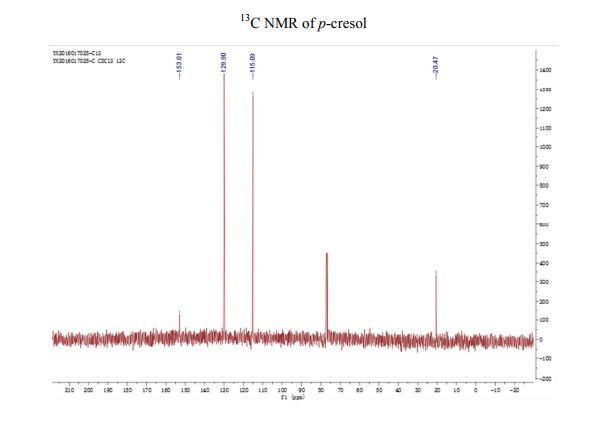
A Fully Continuous-Flow Process for the Synthesis of p-Cresol: Impurity Analysis and Process Optimization

A fully continuous-flow diazotization–hydrolysis protocol has been developed for the preparation of p-cresol. This process started from the diazotization of p-toluidine to form diazonium intermediate. The reaction was then quenched by urea and subsequently followed by a hydrolysis to give the final product p-cresol. Three types of byproducts were initially found in this reaction sequence. After an optimization of reaction conditions (based on impurity analysis), side reactions were eminently inhibited, and a total yield up to 91% were ultimately obtained with a productivity of 388 g/h. The continuous-flow methodology was used to avoid accumulation of the highly energetic and potentially explosive diazonium salt to realize the safe preparation for p-cresol.
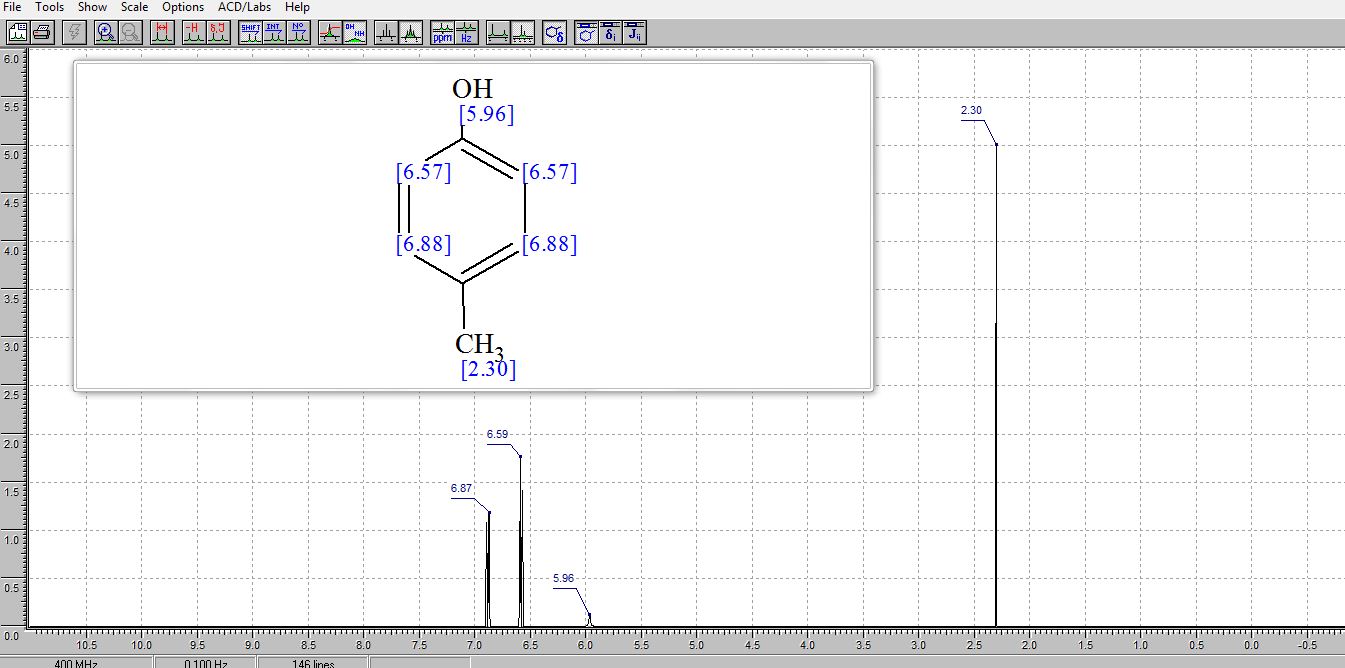
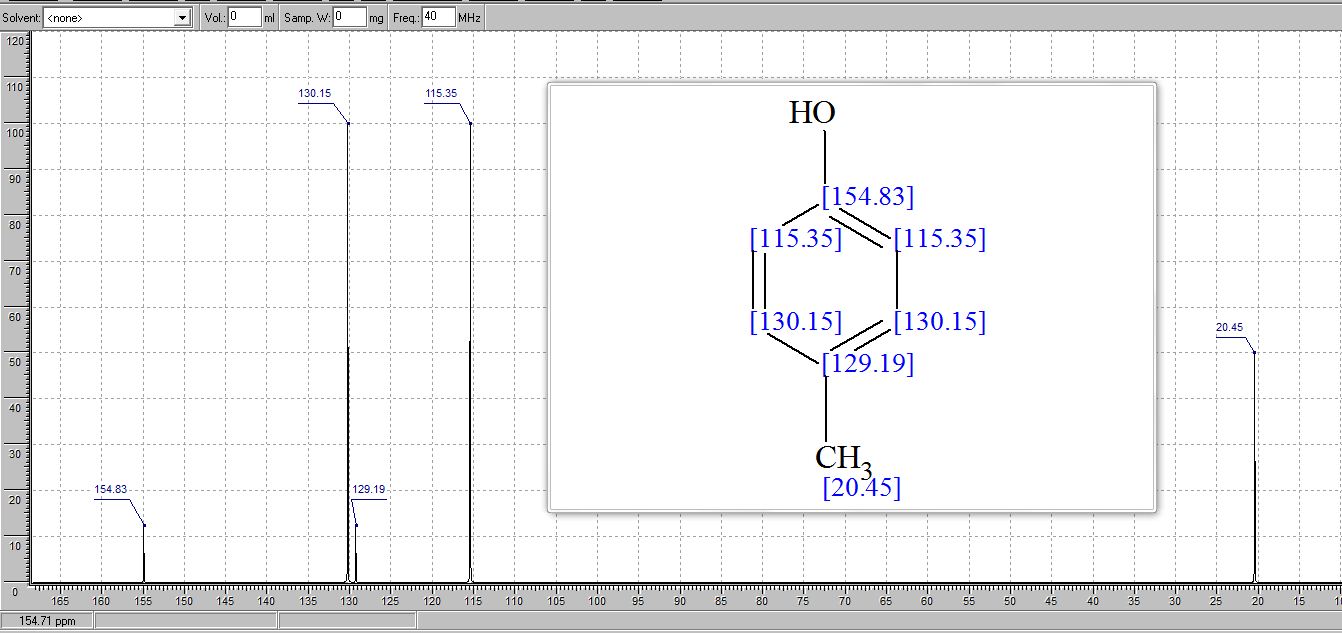
. 1H NMR (400 MHz, (CD3)2SO) δ/ppm: 9.06 (br s, 1H, −OH), 6.94 (d, J = 8.0 Hz, 2H, Ar–H), 6.62 (d, J = 8.0 Hz, 2H, Ar–H), 2.17 (s, 3H, −CH3).
13C NMR (CDCl3) δ/ppm: 153.0, 129.9, 115.1, 20.5.
Literature data:(3b) 1H NMR (300 MHz, CDCl3) δ/ppm: 7.03 (d, J = 8.2 Hz, 2H), 6.73 (dd, J = 8.2, 2.0 Hz, 2H), 4.75 (s, 1H, OH), 2.27 (s, 3H, CH3).
13C NMR (CDCl3) δ/ppm: 153.2, 130.2, 115.2, 20.6.
3(b) Taniguchi, T.; Imoto, M.; Takeda, M.; Nakai, T.; Mihara, M.; Iwai, T.; Ito, T.; Mizuno, T.; Nomoto, A.; Ogawa, A. Heteroat. Chem. 2015, 26, 411– 416 DOI: 10.1002/hc.21275
A Fully Continuous-Flow Process for the Synthesis of p-Cresol: Impurity Analysis and Process Optimization
†National Engineering Research Center for Process Development of Active Pharmaceutical Ingredients, Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, ‡Key Laboratory for Green Pharmaceutical Technologies and Related Equipment of Ministry of Education, College of Pharmaceutical Sciences, Zhejiang University of Technology, Hangzhou 310014, P. R. China
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00250
*Tel.: (+86)57188320899. E-mail: pharmlab@zjut.edu.cn.
NMR PREDICT
Sunday, 1 October 2017
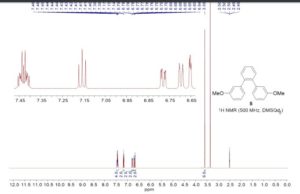
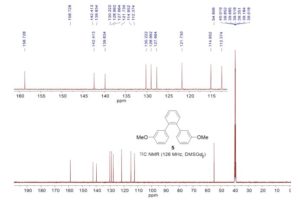
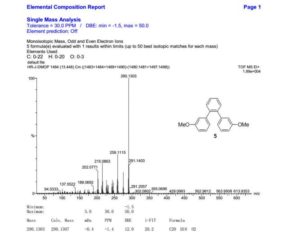
1,2-Bis(3-methoxyphenyl)benzene
1,2-Bis(3-methoxyphenyl)benzene (5)
1H NMR (500 MHz, DMSO-d6): δ 3.59 (s, 6H), 6.65 (dd, J = 2.5, 1.5 Hz, 2H), 6.70 (ddd, J = 7.5, 1.5, 1.0 Hz, 2H), 6.78 (ddd, J= 8.0, 2.5, 1.0 Hz, 2H), 7.16 (t, J = 8.0 Hz, 2H), 7.40–7.46 (m, 4H).
13C NMR (126 MHz, DMSO-d6): δ 54.8, 112.4, 115.0, 121.7, 127.7, 129.0, 130.2, 139.8, 142.4, 158.7.
HRMS (TOF MS EI+) for C20H18O2 [M]+: calcd 290.1307, found 290.1303.
Efficient and Practical Synthesis of Electron Transport Material and Its Key Intermediate
Xiangdong Zhao†, Guijie Li*†  , Zhi-Qiang Zhu‡, Kun Fang†, Yuning Yang†, Jian Li‡
, Zhi-Qiang Zhu‡, Kun Fang†, Yuning Yang†, Jian Li‡  , and Yuanbin She*†
, and Yuanbin She*†
† State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology, College of Chemical Engineering, Zhejiang University of Technology, 18 Chaowang Road, Hangzhou, Zhejiang 310014, P. R. China
‡ Department of Materials Science and Engineering, Arizona State University, Tempe, Arizona 85284, United States
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00280
*E-mail: guijieli@zjut.edu.cn., *E-mail: sheyb@zjut.edu.cn.
Abstract
An efficient and practical synthesis of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)triphenylene 4 from two cheap commodity chemicals in five steps with a total yield of 48.6% was developed. This process had been successfully applied in the synthesis of electron transport material (ETM) BPyTP-2 in the gram scale with a total yield of 47.2%. This practical development of the key intermediate 4 opens a door in its further application in the synthesis of other triphenylene-based ETMs and host materials in the materials field.
/////////////http://pubs.acs.org/doi/10.1021/acs.oprd.7b00280
Thursday, 28 September 2017
Development of a General Protocol To Prepare 2H-1,3-Benzoxazine Derivatives
2H-1,3-Benzoxazine natural products and related bioactive molecules.
4-(2-Bromo-5-chlorobenzyl)-7-chloro-2-phenyl-2H-benzo[e][1,3]oxazine 2 as a light-yellow solid (82% yield).
1H NMR (500 MHz, CDCl3): δ (ppm) 7.55–7.52 (m, 3H), 7.42–7.34 (m, 3H), 7.30 (d, 1H, J = 3.5 Hz,) 7.29 (s, 1H), 7.12 (dd, 1H, J = 8.5 Hz, 2.5 Hz), 6.95–6.91 (m, 2H), 6.57 (1H, s), 4.16 (ABq, 2H, ΔδAB = 0.05, JAB = 16.5 Hz).
13C NMR (125 MHz, CDCl3): δ (ppm) 161.5, 155.8, 139.2, 138.9, 138.1, 133.8, 133.5, 130.6, 128.8, 128.7, 128.5, 127.0, 126.3, 122.6, 121.9, 117.3, 116.2, 88.9, 40.8.
HRMS TOF MS (m/z): [M + H]+ calcd for [C21H14BrCl2NO H] 445.9709; found 445.9713.
FTIR(neat): 3060, 1633, 1596, 1454, 1364, 1344 cm–1.
Spectroscopic data for 2 were identical to those reported in the literature.(4)
Li, H.; Belyk, K. M.; Yin, J.; Chen, Q.; Hyde, A.; Ji, Y.; Oliver, S.; Tudge, M.; Campeau, L.-C.; Campos, K. R. J. Am. Chem. Soc. 2015, 137,13728– 13731 DOI: 10.1021/jacs.5b05934
Development of a General Protocol To Prepare 2H-1,3-Benzoxazine Derivatives
† Department of Process Research and Development, MSD R&D (China) Co., Ltd., Building 21 Rongda Road, Wangjing R&D Base, Zhongguancun Electronic Zone West Zone, Beijing 100012, China
‡ Department of Process Research and Development, Merck Sharp & Dohme, Hertford Road, Hoddesdon, Hertfordshire EN11 9BU, United Kingdom
§ Department of Synthetic Chemistry, Pharmaron Beijing Co., Ltd., 6 Taihe Road BDA, Beijing, 100176, China
∥ Department of Process Research and Development, Merck Research Laboratories, P.O. Box 2000, Rahway, New Jersey 07065, United States
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00209
Publication Date (Web): August 23, 2017
Copyright © 2017 American Chemical Society
*E-mail: ji_qi@merck.com.
ACS Editors’ Choice – This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes.
Abstract
A practical synthesis and detailed development process of 2H-1,3-benzoxazine derivatives catalyzed by aldimine and trifluoromethanesulfonic acid is described. A broad range of substrates with diverse steric and electronic properties were explored. Aliphatic/aromatic/heteroaromatic substrates all proceed well under conditions which have been optimized into a robust, scalable process.
//////////
Wednesday, 27 September 2017
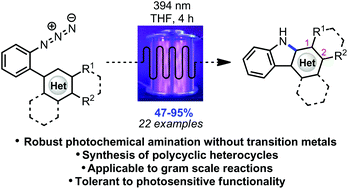
Photochemical intramolecular amination for the synthesis of heterocycles
Green Chem., 2017, Advance Article
DOI: 10.1039/C7GC02261A, Communication
DOI: 10.1039/C7GC02261A, Communication
Shawn Parisien-Collette, Corentin Cruche, Xavier Abel-Snape, Shawn K. Collins
Polycyclic heterocycles can be formed in good to excellent yields via photochemical conversion of the corresponding substituted aryl azides under irradiation with purple LEDs in a continuous flow reactor.
Polycyclic heterocycles can be formed in good to excellent yields via photochemical conversion of the corresponding substituted aryl azides under irradiation with purple LEDs in a continuous flow reactor.
Photochemical intramolecular amination for the synthesis of heterocycles
Author affiliations
*Corresponding authors
aShawn Parisien-Collette, Corentin Cruché, Xavier Abel-Snape and Prof, Dr Shawn K. Collins, Department of Chemistry and Centre in Green Chemistry and Catalysis, Université de Montréal, CP 6128 Station Downtown, Montréal, Canada H3C 3J7
E-mail: shawn.collins@umontreal.ca
E-mail: shawn.collins@umontreal.ca
Abstract
Polycyclic heterocycles can be formed in good to excellent yields via photochemical conversion of the corresponding substituted aryl azides under irradiation with purple LEDs in a continuous flow reactor. The experimental set-up is tolerant to UV-sensitive functional groups while affording diverse carbazoles, as well as an indole and pyrrole framework, in short reaction times. The photochemical method is presumed to progress through a mechanism differing from the other methods of azide activation involving transition metal catalysis.

Methyl 9H-carbazole-2-carboxylate (9): Following the Photodecomposition Procedure A, starting from Methyl 2’-azido-[1,1’-biphenyl]-4-carboxylate, the crude mixture was purified by silica gel column chromatography (100 % hexanes → 10 % ethyl acetate in hexanes), to afford the desired product as a white solid (24.3 mg, 72 % yield). Following the Photodecomposition Procedure B, starting from Methyl 2’-azido-[1,1’- biphenyl]-4-carboxylate, the crude mixture was purified by silica gel column chromatography (100 % hexanes → 10 % ethyl acetate in hexanes), to afford the desired product as a white solid (27.7 mg, 82 % yield). NMR data was in accordance with what was previously reported.16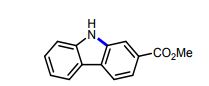
16 Takamatsu, K.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2014, 16, 2892-2895
NEXT..............
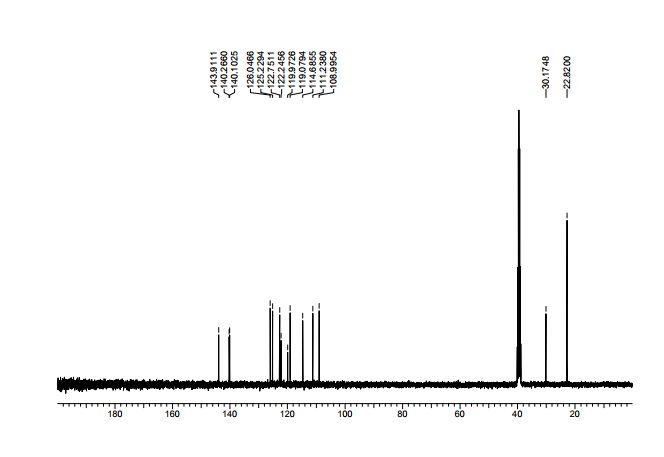
4-Isopropyl-9H-carbazole (14): Following the Photodecomposition Procedure A, starting from 2-azido-2’-isopropyl-1,1’-biphenyl, the crude mixture was purified by silica gel column chromatography (100 % hexanes → 10 % ethyl acetate in hexanes), to afford the desired product as a yellow solid (16.6 mg, 53 % yield). Following the Photodecomposition Procedure B, starting from 2-azido-2’-isopropyl-1,1’-biphenyl and using ethyl acetate as the solvant, the crude mixture was purified by silica gel column chromatography (100 % hexanes → 10 % ethyl acetate in hexanes), to afford the desired product as a yellow solid (16.0 mg, 51 % yield).
1H NMR (400 MHz, DMSO-d6) δ = 11.29 (s, 1H), 8.11 (d, J = 8.1 Hz, 1H), 7.50 (d, J = 8.1 Hz, 1H), 7.39-7.31 (m, 3H), 7.19- 7.15 (m, 1H), 7.08-7.03 (m, 1H), 3.92-3.82 (m, 1H), 1.41 (d, J = 6.8 Hz, 6H);
13C NMR (100 MHz, DMSO-d6) δ = 143.9, 140.3, 140.1, 126.0, 125.2, 122.8, 122.2, 119.9, 119.1, 114.9, 111.2, 108.9, 30.2, 22.8 (2C);
HRMS (ESI) m/z calculated for C15H15N [M-H]- 208.1130; found 208.1126.
Subscribe to:
Comments (Atom)