Bromoclenbuterol
| Bromoclenbuterol; CAS 37153-52-9; Chlorbrombuterol; AC1MC7W8; |
| Molecular Formula: | C12H18BrClN2O |
|---|
| Molecular Weight: | 321.64112 g/mol |
|---|
CLIP
Wide-range screening of banned veterinary drugs in urine by ultra high liquid chromatography coupled to high-resolution mass spectrometry
- a Center for Public Health Research (CSISP), Avda de Cataluña 21, 46020 Valencia, Spain
- b Thermo Fisher Scientific, Barcelona, Spain
- c Analytical Chemistry Department, Universidad de Valencia, Edifici Jeroni Muñoz, 50, Dr. Moliner, 46100 Burjassot, Valencia, Spain
CLIP
Synthesis and Characterization of Bromoclenbuterol
Ravi Kumar Kannasani*, Srinivasa Reddy Battula, Suresh Babu Sannithi, Sreenu Mula and Venkata Babu VV
R&D Division, RA Chem Pharma Limited, API, Hyderabad, Telangana, India
- *Corresponding Author:
- Ravi Kumar Kannasani
R&D Division, RA Chem Pharma Limited
API, Prasanth Nagar, Hyderabad, Telangana, India
Tel: +919000443184
E-mail: kannasani.ravi@rachempharma.com
Citation: Kannasani RK, Battula SR, Sannithi SB, Mula S, Babu VVV (2016) Synthesis and Characterization of Bromoclenbuterol. Med Chem (Los Angeles) 6:546-549. doi:10.4172/2161-0444.1000397
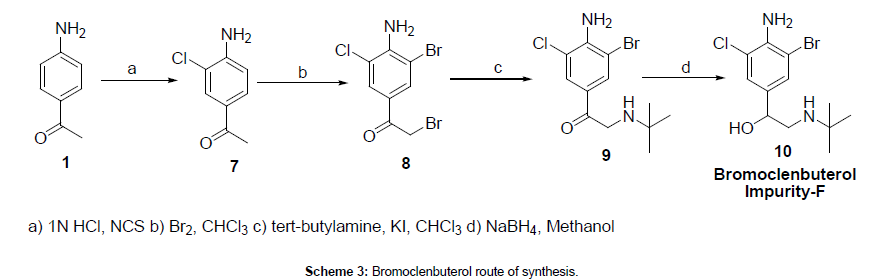
4-Amino acetophenone (1) was reacted with N-Chlorosuccinimide in 1N HCl to afford 4-amino-3-chloro acetophenone (7), which was reacted with bromine to give 1-(4-amino-3-bromo-5-chlorophenyl)- 2-bromoethanone (8). The obtained bromo compound was reacted with tertiay -butyl amine to afford 2-(tert-butylamino)-1-(4-amino-3- bromo-5-chlorophenyl)ethanone (9), which was reduced with sodium borohydride in methanol to give bromoclenbuterol compound (10). The synthesized bromoclenbuterol structure was confirmed by 1H NMR, 13C NMR, IR and mass spectra.
1-(4-Amino-3-chlorophenyl)ethanone (7)
To a stirred solution of 1N HCl (1500 ml) was added 4-amino acetophenone (1) (200 gm, 1.48 mole) and N-Chloro succinimide (50 gm, 0.37 mole) at room temperature, and stirring continued for 3 hrs at 25-30°C. After maintenance undissolved material was filtered from the reaction mixture, total filtrate was taken and extracted with
ethyl acetate, dried over sodium sulfate and evaporated under vacuum to get crude. Crude material was dissolve in ethyl acetate, titrated with EA-HCl and stirred for 15-30 min to get precipitation. The obtained precipitate was filtered and washed with ethyl acetate, and this acidic titration operation was repeated 2 times to get mono chloro compound as solid material, this solid material was
neutralized with sodium carbonate solution in aqueous condition and further purified by using recrystlliaztion technique in ethyl acetate to get 68 gm (yield-27%) 3-chloro-4-amino acetophenone (
7) (mono chloro compound), as light brown colored solid with 98.66% HPLC purity (124 gm of unreacted 4-amino acetophenone obtained from aqueous layer).
1-(4-Amino-3-bromo-5-chlorophenyl)-2-bromoethanone (8)
To a stirred solution of 3-chloro-4-amino acetophenone (7) (14 gm, 0.082 mole) in
chloroform (140 ml) was added bromine (26.24 gm, 0.164 mole) solution slowly at 25-30°C and stirring continued for 6 hrs at same temperature. After completion of the reaction,
methanol was added to the reaction mixture and continued the stirring for 30 min at RT. Undissolved material was filtered, the filtrate was distilled up to 50%, remaining mass was cooled to 0-5°C and filtered to give 15 gm (yield-55%) of 1-(4-amino -3-chloro-5-bromo - phenyl) -2-bromo ethanone (8) as light brown color solid with 95.15% HPLC purity.
2-(Tert-butylamino)-1-(4-amino-3-bromo-5-chlorophenyl) ethanone (9)
To a stirred solution of 1-(4-amino -3-chloro-5-bromo - phenyl) -2-bromo ethanone (8) (8 gm, 0.024 mole) in chloroform (50 ml) was added catalytic amount of potassium iodide (0.1 gm, 0.0006 mole) and tertiary butyl amine (5.2 gm, 0.072 mole) at 0-5°C and stirring was continued for 24 hrs at 0-5°C. After completion of the reaction, undissolved salts were filtered, the filtrate was distilled under
vacuum to get crude solid material, which was triturated with hexane to give 6 gm (yield-76%) of 1-(4-amino-3-chloro-5-bromo phenyl)-2-[(1,1- dimethylethyl)amino]ethanone (9) as light pale yellow color solid.
(S)-2-(Tert-butylamino)-1-(4-amino-3-bromo-5- chlorophenyl)ethanol (10)
To a stirred solution of 1-(4-Amino-3-chloro-5-bromo phenyl)- 2-[(1,1-dimethylethyl)amino]ethanone (9) (6 gm, 0.018 mole) in methanol (25 ml) was added sodium borohydride (0.7 gm, 0.018 mole) at 0-5°C. After addition, reaction mixture was slowly allowed to come to room temperature and stirred for 10 hrs at 25-30°C. On completion, reaction mixture was poured in to chilled water, obtained precipitate was filtered, dried and recrystallized in methanol to give 5 gm (yield-82%) of 1RS-1-(4-amino -3-bromo-5-chloro phenyl) -2-[(1,1-dimethyl ethyl)amino ethanol (or) Bromo clenbuterol (10) as off-white solid. HPLC purity-98.80%,
1H NMR (CDCl3): δ 7.35 (d, 1H, J=1.2 Hz), 7.23 (d, 1H, J=1.6 Hz), 4.45 (br s, 2H), 4.42 (dd, 1H, J=9.2, 3.6 Hz), 2.84 (dd, 1H, J=11.6, 3.6 Hz), 2.50 (dd, 1H, J=12.0, 9.2 Hz), 1.10 (s, 9H).
13C NMR (CDCl3): 140.12, 133.93, 128.46, 126.05, 119.16, 109.08, 70.94, 50.33, 50.05, 29.15.
IR (KBr, Cm-1): 3465.99, 3320.19, 2965.04, 1623.40, 1483.88, 1219.17, 758.77, 630.41.
Mass: (m/z)-323.01 (M+2 peak).
References
- International Conference on Harmonization (ICH) Guidelines (2002) Q3A (R) Impurities in New Drug Substances.
- ICH (2006) Impurities in New Drug Products. Q3B (R1).
- Srinivasulu R, Ravi Kumar K, Nageswararao K (2016) Synthesis of 3,4-dihydropyrimidin-2(1H)-ones using camphor sulfonic acid as a catalyst under solvent-free conditions. Derpharma chemica 8: 375-379.
- Antuna AZ, Ivan L, Pablo RG, Julio R, Jose, et al. (2011) V. Tetrahedron 67: 5577-5581.
- Ehrhardt JD (1990) Synthesis of nonadeutero-clenbuterol. Journal of labeled compounds and radiopharmaceuticals 28: 725-729.
- Drabb TW (1990) Method for the preparation of 4'-(substituted)-amino-2-(substituted) Amino-3’,5'-dichloroacetophenone and salts thereof Trenton. NJ Patent: US4906781A.
- Bentley TJ (1984) Method for the preparation of 1-(4'-amino-3',5'-dichlorophenyl)-2-alkyl(or dialkyl)aminoethanols. US4461914 A.
////////////