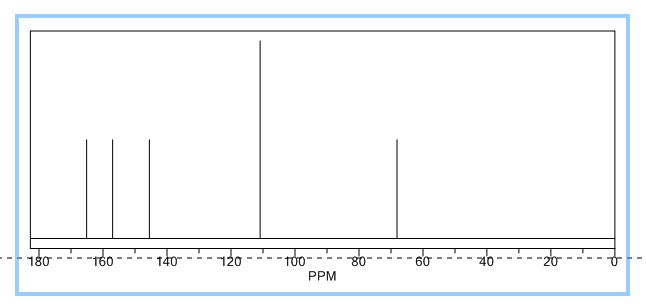
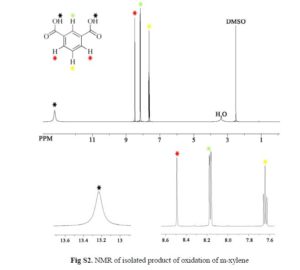
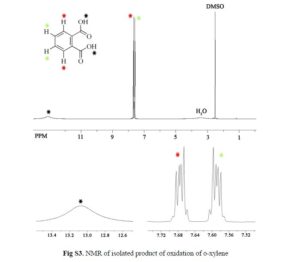
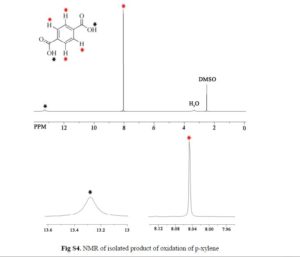
Nanoscale graphene oxide sheets (NGO), as activated carbocatalysts, were synthesized by a reduction size strategy and used in chemoselective oxidative conversion of benzylic C–H to the corresponding carboxylic acid. On the basis of the results of the optimization process of different parameters, 3 equiv of H2O2 for each C–H group, 100 wt % of NGO in aqueous medium, acetone as a cosolvent, and a reaction temperature of 100 °C were selected as optimum parameters.
In this optimum condition, xylenes and toluene over 24 h with good yield were converted to the corresponding carboxylic acid, and in the case of diphenylmethane and ethylbenzene, these substrates with excellent yield were converted to benzophenone and acetophenone.



Selective Oxidation of Benzylic C–H Using Nanoscale Graphene Oxide as Highly Efficient Carbocatalyst: Direct Synthesis of Terephthalic Acid
Institute of Industrial Chemistry, Iranian Research Organization for Science and Technology (IROST), P.O. Box 33535-111, Tehran, Iran
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00056
*Tel.: +98 21 56276031. E-mail address: sedrpoushan1395@gmail.com (Alireza Sedrpoushan).
///////



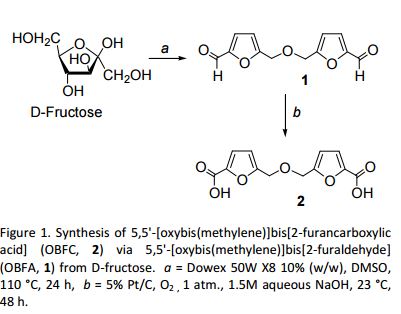
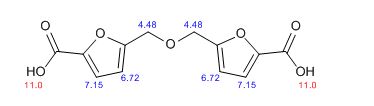
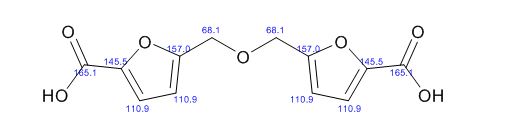
![Graphical abstract: A two-step efficient preparation of a renewable dicarboxylic acid monomer 5,5′-[oxybis(methylene)]bis[2-furancarboxylic acid] from d-fructose and its application in polyester synthesis](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C6GC03314H)