INCB-39110,
CAS 1334298-90-6
INCB-039110, Jak1 tyrosine kinase inhibitor
- 3-Azetidineacetonitrile, 1-[1-[[3-fluoro-2-(trifluoromethyl)-4-pyridinyl]carbonyl]-4-piperidinyl]-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]-
C26H23F4N9O (MW, 553.51)
{ l- { l-[3-fluoro-2- (trifluoromethyl)isonicotinoyl]piperidin-4-yl}-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4- yl)-lH-pyrazol-l-yl]azetidin-3-yl}acetonitrile
2-(3-(4-(7H-pyrrolo[2,3-( Jpyrimidin-4-yl)-lH- pyrazol- 1 -yl)- 1 -( 1 -(3 -fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin- 3-yl)acetonitrile
2-(3-(4-(7H- Pyrrolo[2,3 -i/]pyrimidin-4-yl)- lH-pyrazol- 1 -yl)- 1 -(1 -(3 -fluoro-2- (trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile adipateMAY BE THE DRUG… HAS CAS 1334302-63-4


ADIPATE OF INCB-39110
ALSO/OR
3-Azetidineacetonitrile, 1-[1-(3-fluorobenzoyl)-4-methyl-4-piperidinyl]-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]-, 2,2,2-trifluoroacetateMAY BE THE DRUG ????… HAS CAS 1334300-52-5
US 2011/0224190 is the pdt patent

base
smilesN#CCC6(n3cc(c1ncnc2[n]ccc12)cn3)CN(C5CCN(C(=O)c4ccnc(C(F)(F)F)c4F)CC5)C6
free base (22, 7.00 g, 93.5%) as an off-white solid. For 22:
1H NMR (400 MHz, (CD
3)
2SO) δ 12.17 (d, J=2.8 Hz, 1H), 8.85 (s, 1H), 8.70 (m, 2H), 8.45 (s, 1H), 7.93 (t, J=4.7 Hz, 1H), 7.63 (dd, J=3.6, 2.3 Hz, 1H), 7.09 (dd, J=3.6, 1.7 Hz, 1H), 4.10 (m, 1H), 3.78 (d, J=7.9 Hz, 2H), 3.61 (t, J=7.9 Hz, 1H), 3.58 (s, 2H), 3.46 (m, 1H), 3.28 (t, J=10.5 Hz, 1H), 3.09 (ddd, J=13.2, 9.5, 3.1 Hz, 1H), 2.58 (m, 1H), 1.83-1.75 (m, 1H), 1.70-1.63 (m, 1H), 1.35-1.21 (m, 2H) ppm;
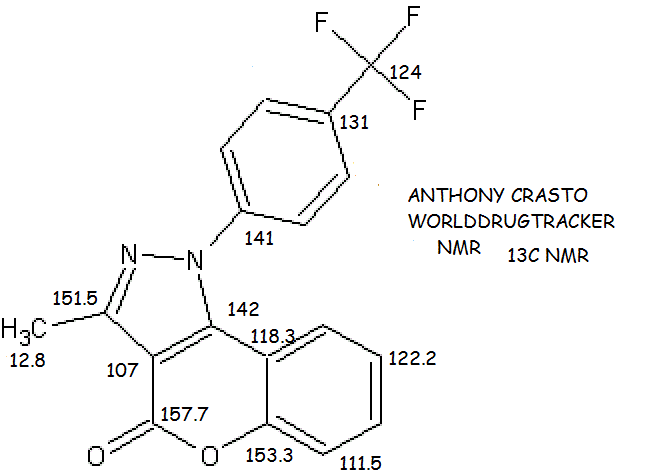
13C NMR (101 MHz, (CD3)2SO) δ 160.28, (153.51, 150.86), 152.20, 150.94, 149.62, (146.30, 146.25), 139.48, (134.78, 134.61), (135.04, 134.92, 134.72, 134.60, 134.38, 134.26, 134.03, 133.92), 129.22, 127.62, 126.84, 121.99, 122.04, (124.77, 122.02, 119.19, 116.52), 117.39, 113.00, 99.99, 61.47, 60.49, 57.05, 44.23, 28.62, 27.88, 27.19 ppm;
C26H23F4N9O (MW, 553.51), LCMS (EI) m/e 554.1 (M′+H).
ADIPATE
Example 8
2-(3-(4-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(1-(3-fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile adipate (25)
Step 1. 2-(3-(4-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(1-(3-fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile adipate crude salt (24)
The process of making compound 22 in Example 7 was followed, except that the final organic phase was concentrated by vacuum distillation to the minimum volume to afford crude compound 22, which was not isolated but was directly used in subsequent adipate salt formation process. To the concentrated residue which containing crude compound 22 was added methanol (200 mL) at room temperature. The mixture was the concentrated by vacuum distillation to a minimum volume. The residue was then added methanol (75 mL) and the resulting solution was heated to reflux for 2 hours. Methyl isobutyl ketone (MIBK, 75 mL) was added to the solution and the resulting mixture was distilled under vacuum to about 30 mL while the internal temperature was kept at 40-50° C. Methanol (75 mL) was added and the resulting mixture was heated to reflux for 2 hours. To the solution was added MIBK (75 mL). The mixture was distilled again under vacuum to about 30 mL while the internal temperature was kept at 40-50° C. To the solution was added a solution of adipic acid (23, 2.15 g, 14.77 mmol) in methanol (75 mL). The resultant solution was then heated to reflux for 2 hours. MIBK (75 mL) was added. The mixture was distilled under vacuum to about 60 mL while the internal temperature was kept at 40-50° C. Heating was stopped and heptane (52.5 mL) was added over 1-2 hours. The resultant mixture was stirred at 20±5° C. for 3-4 hours. The white precipitates were collected by filtration, and the filter cake was washed with heptane (2×15 mL). The solid was dried on the filter under nitrogen with a pulling vacuum at 20±5° C. for 12 hours to provide compound 24 (crude adipate salt, 8.98 g, 12.84 mmol., 95.0%). For 24: 1H NMR (400 MHz, (CD3)2SO) δ 12.16 (s, 1H), 12.05 (brs, 2H), 8.85 (s, 1H), 8.72 (s, 1H), 8.69 (d, J=4.7 Hz, 1H), 8.45 (s, 1H), 7.93 (t, J=4.7 Hz, 1H), 7.63 (dd, J=3.6, 2.3 Hz, 1H), 7.09 (dd, J=3.6, 1.7 Hz, 1H), δ 4.11 (dt, J=11.0, 4.4 Hz, 1H), 3.77 (d, J=7.8 Hz, 2H), 3.60 (t, J=7.8 Hz, 2H), 3.58 (s, 2H), 3.44 (dt, J=14.4, 4.6 Hz, 1H), 3.28 (t, J=10.4 Hz, 1H), 3.09 (ddd, J=13.2, 9.6, 3.2 Hz, 1H), 2.58 (tt, J=8.6, 3.5 Hz, 1H), 2.28-2.17 (m, 4H), 1.83-1.74 (m, 1H), 1.67 (d, J=11.0 Hz, 1H), 1.59-1.46 (m, 4H), 1.37-1.21 (m, 2H) ppm; 13C NMR (101 MHz, (CD3)2SO) δ 174.38, 160.29, (153.52, 150.87), 152.20, 150.94, 149.63, (146.30, 146.25), 139.48, (134.79, 134.62), (135.08, 134.97, 134.74, 134.62, 134.38, 134.28, 134.04, 133.93), 129.21, 127.62, 126.84, 122.05, (124.75, 122.02, 119.29, 116.54), 117.39, 113.01, 99.99, 61.47, 60.50, 57.06, 44.24, 33.42, 30.70, 28.63, 27.89, 27.20, 24.07 ppm; C32H33F4N9O5 (Mol. Wt: 699.66; 24: C26H23F4N9O, MW 553.51), LCMS (EI) m/e 554.0 (M++H).
Step 2.
2-(3-(4-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(1-(3-fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile adipate (25)
In a 100 L dried reactor equipped with a mechanical stirrer, a thermocouple, an addition funnel and a nitrogen inlet was added compound 24 (3.40 kg, 4.86 mol) and acetone (23.8 L). The resulting white turbid was heated to 55-60° C. to provide a clear solution. The resultant solution was filtered through an in-line filter to another 100 L reactor. Heptane (23.8 L) was filtered through an in-line filter to a separated 50 L reactor. The filtered heptane was then charged to the acetone solution in the 100 L reactor at a rate while the internal temperature was kept at 55-60° C. The reaction mixture in the 100 L reactor was then cooled to 20±5° C. and stirred at 20±5° C. for 16 hours. The white precipitates were collected by filtration and the cake was washed with heptane (2×5.1 L) and dried on the filter under nitrogen with a pulling vacuum. The solid was further dried in a vacuum oven at 55-65° C. with nitrogen purge to provide compound 25 (3.11 kg, 92.2%) as white to off-white powder. For 25:
ADIPATE OF INCB 39110
1H NMR (400 MHz, (CD3)2SO) δ 12.16 (s, 1H), 12.05 (brs, 2H), 8.85 (s, 1H), 8.72 (s, 1H), 8.69 (d, J=4.7 Hz, 1H), 8.45 (s, 1H), 7.93 (t, J=4.7 Hz, 1H), 7.63 (dd, J=3.6, 2.3 Hz, 1H), 7.09 (dd, J=3.6, 1.7 Hz, 1H), δ 4.11 (dt, J=11.0, 4.4 Hz, 1H), 3.77 (d, J=7.8 Hz, 2H), 3.60 (t, J=7.8 Hz, 2H), 3.58 (s, 2H), 3.44 (dt, J=14.4, 4.6 Hz, 1H), 3.28 (t, J=10.4 Hz, 1H), 3.09 (ddd, J=13.2, 9.6, 3.2 Hz, 1H), 2.58 (tt, J=8.6, 3.5 Hz, 1H), 2.28-2.17 (m, 4H), 1.83-1.74 (m, 1H), 1.67 (d, J=11.0 Hz, 1H), 1.59-1.46 (m, 4H), 1.37-1.21 (m, 2H) ppm;
13C NMR (101 MHz, (CD3)2SO) δ 174.38, 160.29, (153.52, 150.87), 152.20, 150.94, 149.63, (146.30, 146.25), 139.48, (134.79, 134.62), (135.08, 134.97, 134.74, 134.62, 134.38, 134.28, 134.04, 133.93), 129.21, 127.62, 126.84, 122.05, (124.75, 122.02, 119.29, 116.54), 117.39, 113.01, 99.99, 61.47, 60.50, 57.06, 44.24, 33.42, 30.70, 28.63, 27.89, 27.20, 24.07 ppm;
C32H33F4N9O5 (Mol. Wt: 699.66; free base: C26H23F4N9O (MW, 553.51), LCMS (EI) m/e 554.0 (M++H).