LAPATINIB
Title: Lapatinib
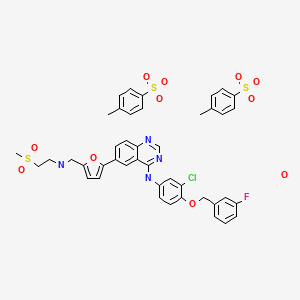
- N-(3-Chloro-4-((3-fluorobenzyl)oxy)phenyl)-6-(5-(((2-(methylsulfonyl)ethyl)amino)methyl)furan-2-yl)quinazolin-4-amine bis(4-methylbenzenesulfonate) monohydrate
Preparation of Lapatinib Ditosylate
To a stirred mixture of Sodiumtriacetoxyborohydride (0.21 g) in Tetrahydrofuran (THF)(2.4 ml) was added N-(3-Chloro-4-(3-fluorobenzyloxy)phenyl)-6-(5-((2- (methylsulfonyl)ethylimino)- methyl)furan-2-yl)quinazolin-4-amine (0.2 g) in THF. The reaction mixture was stirred for 1 hour at 20-25 °C. Reaction was monitored by TLC and on completion of reaction, aqueous NaQH (0.16 g NaOH to 0.8 g demineralized water) was added. The organic layer was separated and added p- Toluenesulfonic acid (0.42) in THF (0.6 ml) and stirred for 3 hours. The solid was filtered and dried under vacuum at 60-65°C till constant weight.
Weight: 0.15 g
Yield: 46.9 %
Purity by HPLC: 96.16%
MS (ES+) m/z: 581 [M+H]+ & 583 [M+H+2]+
1H NMR (400 MHz; DMSO-d6): 2.28 (s, 6H), 3.14 (s, 3H), 3.44 (t, J=8.0 Hz, 2H), 3.55 (t, J=8.0 Hz, 2H), 4.46 (s, 2H), 5.31 (s, 2H), 6.89 (br s, 1H), 7.10 (d, J=7.2 Hz, 4H), 7.20 (m, 1H), 7.23 (br s, 1H), 7.31- 7.36 (m, 3H), 7.47 (d, J=7.2 Hz, 4H), 7.63 (d, J=8.8 Hz, IH), 7.89 (br s, IH), 7.92 (d, J=8.8 Hz, IH), 8.39 (d, J=8.8 Hz, IH), 8.89 (s, IH), 8.98 (s, IH), 9.28 (s, IH, NH), 11.18 (s, IH, NH).
Fresenius Kabi Oncology Ltd.WO 2013080218
Lahiri, Saswata; Gupta, Nitin; Singh, Hemant Kumar; Handa, Vishal; Sanghani, Sunil
6 JUNE 2013, http://www.google.com/patents/WO2013080218A1?cl=en
No comments:
Post a Comment