2-(3-oxobutyl)cyclopentanone-2-carboxylic acid ethyl ester
1H NMR (400 MHz, CDCl3) δ: 1.23 (t, 3 H, J = 7.2), 1.82 – 2.03 (m, 4 H), 2.03 – 2.13 (m, 1 H), 2.12 (s, 3 H), 2.24 – 2.49 (m, 4 H), 2.69 (ddd, 1 H, J = 18, J = 9.6, J = 6.0), 4.14 (q, 2 H, J = 7.1) ;
| 1H-NMR: 2-(3-Oxobutyl)cyclopentanone-2-carboxylic acid ethyl ester |
| 300 MHz, CDCl3 |
| delta [ppm] | mult. | atoms | assignment |
| 1.23 | t | 3 H | CH3 ethyl |
| 1.82-2.13 | m | 5 H | 3-H, 4-H (ring), 1-Ha (3-oxobutyl) |
| 2.12 | s | 3 H | 4-H (CH3 3-oxobutyl) |
| 2.24-2.49 | m | 4 H | 5-H (ring), 2-H (3-oxobutyl) |
| 2.69 | ddd | 1 H | 1-Hb (3-oxobutyl) |
| 4.14 | q | 2 H | CH2 ethyl |
13C NMR (50 MHz, CDCl3) δ: 13.29 (CH3), 18.84 (CH2), 26.24 (CH2), 29.00 (CH3), 33.22 (CH2), 37.07 (CH2), 38.01 (CH2), 58.23 (C), 60.23 (OCH2), 170.47 (C=O), 206.61 (C=O), 213.75 (C=O) .
| 13C-NMR: 2-(3-Oxobutyl)cyclopentanone-2-carboxylic acid ethyl ester |
| 75.5 MHz, CDCl3 |
| delta [ppm] | assignment |
| 13.79 | CH3 ethyl |
| 19.29 | C4 (ring) |
| 26.74 | C5 (ring) |
| 29.59 | C4 (3-oxobutyl) |
| 33.97 | C1 (3-oxobutyl) |
| 37.66 | C2 (3-oxobutyl) |
| 38.58 | C3 (ring) |
| 58.70 | C1 (ring) |
| 61.10 | CH2 ethyl |
| 171.07 | -C(=O)O- |
| 207.37 | C3 (C=O, 3-oxobutyl) |
| 214.46 | C2 (C=O, ring) |
| 76.5-77.5 | CDCl3 |
IR (ATR) cm−1: 2976 (m), 1748 (vs), 1717 (vs), 1448 (m), 1406 (m), 1367 (m), 1318 (m), 1260 (s), 1232 (s), 1165 (s), 1116 (m), 1029 (m), 861 (m)
| IR: 2-(3-Oxobutyl)cyclopentanone-2-carboxylic acid ethyl ester |
| [Film, T%, cm-1] |
| [cm-1] | assignment |
| 2976 | aliph. C-H valence |
| 1749 | C=O valence, ester |
| 1719 | C=O valence, ketone |
Synthesis of 2-(3-oxobutyl)cyclopentanone-2-carboxylic acid ethyl ester
| Educts | | Amount | Risk | Safety |
|---|
| Cyclopentanone-2-carboxylic acid ethyl ester |
| 15.6 g | R no data | S 24/25 |
| Methyl vinyl ketone |
 | F |  | T+ |
| 9.81 g | R 11-26/28-34-37-40-43 | S 16-26-28.1-36/37/39-45 |
| Catalyst | | Amount | Risk | Safety |
|---|
| Ferric chloride hexahydrate |
 | Xn |
| 540 mg | R 22-38-41 | S 26-39 |
| Solvents | | Amount | Risk | Safety |
|---|
| tert-Butyl methyl ether |
 | F |  | Xi |
| 40 mL | R 11-38 | S 2-9-16-24 |
| Water |
| 60 mL | R | S |
| Others | | Amount | Risk | Safety |
|---|
| Sodium sulfate |
 | Xi |
| | R 36/37/38 | S 26-36 |
| Solvents for analysis | | Amount | Risk | Safety |
|---|
| Petroleum ether (60-80) |
 | F |
| | R 11-38-48/20-52/53-62-65 | S 9-16-23.2-24-29-33-36/37-62 |
| tert-Butyl methyl ether |
 | F |  | Xi |
| | R 11-38 | S 2-9-16-24 |
| Dichloromethane |
 | Xn |
| | R 40 | S 2-23.1-24/25-36/37 |
| Reaction type: | addition to alkenes, Michael addition |
| Substance classes: | carboxylic acid ester, ketone, acid catalyst |
| Techniques: | working with cover gas, stirring with magnetic stir bar, adding dropwise with an addition funnel, extracting, shaking out, evaporating with rotary evaporator, distilling under reduced pressure, vacuum pump, heating with oil bath |
| |
Instruction (batch scale 100 mmol)
Equipment 50 mL two-neck flask, protective gas supply, addition funnel with pressure balance, heatable magnetic stirrer, magnetic stir bar, separating funnel, rotary evaporator, distillation apparatus, water bath, vacuum pump, oil bath, Substances cyclopentanone-2-carboxylic acid ethyl ester, (bp 224-228 °C) 15.6 g (14.5 mL, 100 mmol) methyl vinyl ketone (distilled) (bp 80-81 °C) 9.81 g (11.5 mL, 140 mmol) iron(III) chloride hexahydrate 0.54 g (2.0 mmol) tert-butyl methyl ether (bp 55 °C) 40 mL sodium sulfate for drying Reaction 15.6 g (14.5 mL, 100 mmol) cyclopentanone-2-carboxylic acid ethyl ester and 0.54 g (2.0 mmol) iron(III) chloride hexahydrate are filled in a 50 mL two-neck flask, for reasons of temperature constance fixed in a spacious water bath at room temperature and equipped with an addition funnel with pressure balance, a magnetic stir bar and a nitrogen connection. The apparatus is thoroughly rinsed with nitrogen. 9.81 g (11.5 mL, 140 mmol) methyl vinyl ketone are added under stirring within one hour. Afterwards the reaction mixture is stirred for further 4 hours at room temperature. The reaction can be monitored either by thin layer chromatography or gas chromatography (see analytics).
Work up The reaction mixture is transferred with 25 mL tert-butyl methyl ether into a separation funnel and shaken out four times with 15 mL water each. The combined aqueous phases are again shaken out with 15 mL tert-butyl methyl ether. The organic phases are combined, dried with sodium sulfate, filtered and the solvent is evaporated at a rotary evaporator. Crude yield: 22.2g The crude product is distilled under reduced pressure over a short distillation bridge. Yield: 18.0 g (79.5 mmol, 80%); head temperature 90°C (3·10-3 hPa, oil bath temperature 120 C), colourless liquid; n 20 D = 1.4658 Comments
The extraction steps during work-up serve the removal of iron salts from the product solution. If one goes without, decomposition products are formed during distillation.
Waste management Recycling tert-Butyl methyl ether is collected and redistilled Waste disposal Waste Disposal distillation residue dissolve in little acetone, then: organic solvents, containing halogen sodium sulfate solid waste, free from mercury, Time 10-11 hours Break Before shaking out and before distillation
Operating scheme
|
 |
|
 | two-necked flask 50 mL | |  | protective gas piping |
 | addition funnel with pressure balance | |  | heatable magnetic stirrer with magnetic stir bar |
 | separating funnel | |  | rotary evaporator |
 | distillation apparatus | |  | water bath |
 | vacuum pump | |  | oil bath |
Simple evaluation indices
|
| Atom economy | | 100 | % |
| Yield | | 80 | % |
| Target product mass | | 18 | g |
| Sum of input masses | | 110 | g |
| Mass efficiency | | 160 | mg/g |
| Mass index | | 6.3 | g input / g product |
| E factor | | 5.3 | g waste / g product |
| GC: crude product |
| column | DB-1, L=28 m, d=0.32 mm, film=0.25 µm |
| inlet | on column injection, 0.2 µL |
| carrier gas | H2, 40 cm/s |
| oven | 90°C (5 min), 10°C/min –> 240°C (30 min) |
| detector | FID, 270°C |
| integration | percent concentration calculated from relative peak area |
| GC: pure product |
| column | DB-1, L=28 m, d=0.32 mm, film=0.25 µm |
| inlet | on column injection, 0.2 µL |
| carrier gas | H2, 40 cm/s |
| oven | 90°C (5 min), 10°C/min –> 240°C (30 min) |
| detector | FID, 270°C |
| integration | percent concentration calculated from relative peak area |
more….

- 2-(3-Oxobutyl)cyclopentanone-2-carboxylic acid ethyl ester, which is known as Cyclopentanecarboxylic acid, 2-oxo-1-(3-oxobutyl)-, ethyl ester, could be produced through the following synthetic method.
 A 50-mL, round-bottomed flask, equipped with a magnetic stirring bar, is charged with cyclopentanone-2-carboxylic acid ethyl ester (25.0 g, 160 mmol) and iron(III)chloride hexahydrate (865 mg, 3.20 mmol). The flask is kept in a water bath at room temperature (external temperature), and methyl vinyl ketone (MVK) (15.0 mL, 12.7 g, 182 mmol) is added within 1 hr using a syringe pump. The resulting mixture is stirred for 12 hr at room temperature, then all volatile materials are removed under reduced pressure from the reaction mixture at room temperature for 3 hr with continued stirring. Subsequently, the flask is equipped with a Claisen top and condenser and the product is distilled under high vacuum. The distillate is collected in a single receiver flask to afford 33.3-33.7 g (91-93%) of analytically pure 2-(3-oxobutyl)cyclopentanone-2-carboxylic acid ethyl ester.
A 50-mL, round-bottomed flask, equipped with a magnetic stirring bar, is charged with cyclopentanone-2-carboxylic acid ethyl ester (25.0 g, 160 mmol) and iron(III)chloride hexahydrate (865 mg, 3.20 mmol). The flask is kept in a water bath at room temperature (external temperature), and methyl vinyl ketone (MVK) (15.0 mL, 12.7 g, 182 mmol) is added within 1 hr using a syringe pump. The resulting mixture is stirred for 12 hr at room temperature, then all volatile materials are removed under reduced pressure from the reaction mixture at room temperature for 3 hr with continued stirring. Subsequently, the flask is equipped with a Claisen top and condenser and the product is distilled under high vacuum. The distillate is collected in a single receiver flask to afford 33.3-33.7 g (91-93%) of analytically pure 2-(3-oxobutyl)cyclopentanone-2-carboxylic acid ethyl ester.
BALI, INDONESIA
en.wikipedia.org/wiki/Bali
Bali is an island and province of Indonesia. The province includes the island of Bali and a few smaller neighbouring islands, notably Nusa Penida, Nusa ...
Kuta, Bali
 |  |
| Downtown Kuta on Bali | Gate to Kuta Beach |
The beach of Kuta stretches from Kuta to the neighboring town of Legian.
 |  |
| Kuta Beach |
 |  |
| Temple in Ubud | Bridge in the Sacred Monkey Sanctuary |
 |  |
| Street in Seminyak | Rice field in Seminyak |
 |  |
| Boats waiting at Perambai | Fisherboat on the way to the Gilis |
 |  |
| Beach on Gili Air | There are no motor vehicles on the Gilis |
 |  |
| Beachfront restaurant on Gili Air | Ordering grilled fresh fish |
 |  |
| Tanah Lot temple on Bali |
 |  |
| Temple procession in Bali | Cliffs at Ulu Watu |






 Ngurah Rai International Airport is located in southern Bali. The capital of Bali, Denpasar, is located nearby.
Ngurah Rai International Airport is located in southern Bali. The capital of Bali, Denpasar, is located nearby.
The airport is named after I Gusti Ngurah Rai, an Indonesian republican who died on 20 November 1946 in a puputan (fight to the death) against the Dutch. The Dutch defeated his company with air support, killing Rai and 95 others during the Indonesian Revolution in 1946. The airport is located in Tuban between Kuta and Jimbaran and is close to the tourist locations of southern Bali; the resort center of Kuta is 2.5 km north of the airport.
A kaki lima food cart serving bakso - a typical streetside scene in Bali
Satay lilit - minced seafood on a lemon grass stick, grilled over charcoal
Preparing for a colorful odalan temple anniversary procession
Cities
- Denpasar — a bustling city, the administrative centre and transport hub of the island but not a major tourist destination
- Candidasa — a quiet coastal town, the Bali Aga and gateway to the east coast
- Kuta — surfer central, by far the most heavily developed area in Bali. Lots of shopping and night-life and the centre of lower-end party culture on Bali
- Jimbaran — close to the airport, sea-side resorts, a nice sheltered beach and seafood restaurants south of Kuta
- Legian — located between Kuta and Seminyak; also the name of Kuta´s main street
- Lovina — beautiful black volcanic sand beaches and coral reefs
- Sanur — sea-side resorts and beaches popular with older families
- Seminyak — quieter, more upscale beachside resorts and villas just to the north of Legian, with some fashionable upscale restaurants and trendy designer bars and dance clubs
- Ubud — the centre of art and dance in the foothills, with several museums, the monkey forest and lots of arts and crafts shops
Regions
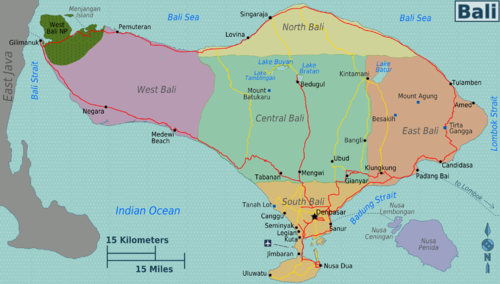
Map of Bali with regions colour coded
| South Bali (Kuta, Bukit Peninsula, Canggu, Denpasar, Jimbaran, Legian, Nusa Dua, Sanur, Seminyak, Tanah Lot)
The most visited part of the island by far, with Kuta Beach and chic Seminyak. |
A 50-mL, round-bottomed flask, equipped with a magnetic stirring bar, is charged with cyclopentanone-2-carboxylic acid ethyl ester (25.0 g, 160 mmol) and iron(III)chloride hexahydrate (865 mg, 3.20 mmol). The flask is kept in a water bath at room temperature (external temperature), and methyl vinyl ketone (MVK) (15.0 mL, 12.7 g, 182 mmol) is added within 1 hr using a syringe pump. The resulting mixture is stirred for 12 hr at room temperature, then all volatile materials are removed under reduced pressure from the reaction mixture at room temperature for 3 hr with continued stirring. Subsequently, the flask is equipped with a Claisen top and condenser and the product is distilled under high vacuum. The distillate is collected in a single receiver flask to afford 33.3-33.7 g (91-93%) of analytically pure 2-(3-oxobutyl)cyclopentanone-2-carboxylic acid ethyl ester.























 Ngurah Rai International Airport is located in southern Bali. The capital of Bali, Denpasar, is located nearby.
Ngurah Rai International Airport is located in southern Bali. The capital of Bali, Denpasar, is located nearby.




Methyl vinyl ketone
ReplyDeleteHow do you get methyl ketone?
Methyl ketones are often directly prepared from carboxylic acids by reaction with methyllithium. Other simple alkyl ketones may also be prepared in the same fashion, making this a method that should be considered whenever these substrates are required.
Methyl vinyl ketone | Manufacturers | Supplier | Exporter | Spain | France | Europe | Switzerland | Italy | LifeChem Pharma
Nice articles and your information valuable and good articles thank for the sharing information Thin film evaporators
ReplyDelete