2,4,6-Tri(propan-2-ylidene)-1,3,5-trioxane
1,3,5-Trioxane-2,4,6-trione (cyclic trimer of CO2)
is the product of a four-step synthesis: chlorination of
isobutyraldehyde; cyclotrimerization of 2-chloro-2-methylpropanal;
dehydochlorination of 2,4,6-tris(2-chloropropan)-2-yl-1,3,5-trioxane;
ozonolysis at −80 °C of 2,4,6-tri(propan-2-ylidene)-1,3,5-trioxane. This
trioxane-trione is detected in solution at temperatures between −80 to
−40 °C, and its conversion to CO2 is monitored by 13C NMR and FTIR. The CO2 trimer has a half-life of approximately 40 min at −40 °C.
As
a product of combustion and respiration whose accumulation in the
atmosphere has become a cause for significant concern, carbon dioxide
has been the subject of much research directed at its reutilization.
Various approaches toward this CO2 reutilization goal have
been described in excellent reviews over the past two decades.Important
processes involve reduction with hydrogen,coupling with other small
molecules, incorporation into polymers and artificial
photosynthesis. The main products include fuels, solvents, chemical
intermediates and polymers.
The efficiency of these commercial processes in terms of reagent usage is relatively low with respect to the fraction of CO2
incorporated into the product; the highest being for urea (57%), and
decreasing for salicylic acid (36%) and methanol (10%). This could be
raised to 100% if a CO2 self-fixation chemistry could be developed. Ideally with a sufficient input of energy, CO2
would react with itself to yield a liquid or solid product from which
this energy could be extracted when needed for useful work. Such
chemistry has been the subject of theoretical calculation for structures
representing the linear polymer and cyclic oligomers of CO2.
With
respect to thermodynamic stability, the cyclic trimer has been
described as “feasible” although energetically less stable than three CO
2 molecules by 27 kJ/mol per CO
2 unit.
(10)Regarding kinetic stability of the cyclic trimer toward fragmentation to CO
2,
calculated barriers for this decomposition have ranged from activation
energies of 61 to 172 kJ/mol depending on the computational method with
calculated half-lives ranging from days to milliseconds at ambient
conditions and substantially longer at lower temperatures.
The cyclic trimer of CO2has also been proposed as a low-energy intermediate in the transformation of CO2 to an extended solid.
The
formation of an orthocarbonate extended covalent structure of
interconnected six-membered rings was predicted by model calculation
with the finding of a stabilization energy that increased with molecular
size. Later experimental work found under extreme pressure/temperature
(40 GPa/1800 K), CO2 will transform to a metastable extended solid which has been characterized as a Phase V form of CO2 with a sigma bonded quartz-like structure.
It has also been proposed that sorption of CO2
into the isolated nanoscale confined spaces of sulfur- or
nitrogen-treated porous carbon at 30 bar pressure can produce a
polymeric structure of carbon dioxide as has been reported for other
molecules in nanoconfined spaces.
The 1,3,5-trioxane-2,4,6-trione structure of the CO2 cyclic trimer, 1, may represent an important intermediate or product in the self-fixation of gaseous CO2.
Theoretical studies on this molecule have indicated a possibility of
kinetic stability at room temperature and as well as a possibility for
it to be thermodynamically feasible.To date, no experimental evidence
has been reported for its existence. The objective of this work is to
synthesize compound 1 and to make an assessment of its stability.
The approach is that of a model compound synthesis where the trioxane
ring is first generated from substituted aldehydes and then the
peripheral carbonyl structures are incorporated at low temperature in
the final step. As will be shown, compound 1does not possess the stability for facile isolation and storage
Synthesis and Low Temperature Spectroscopic Observation of 1,3,5-Trioxane-2,4,6-Trione: The Cyclic Trimer of Carbon Dioxide
† Chemistry Division, Naval Research Laboratory, Washington, D. C. 20375, United States
§Mettler-Toledo AutoChem, Inc., Columbia, Maryland 21046, United States
J. Org. Chem., Article ASAP
DOI: 10.1021/acs.joc.6b00647
ACS Editors' Choice - This is an open access article published under an ACS AuthorChoice
License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes.
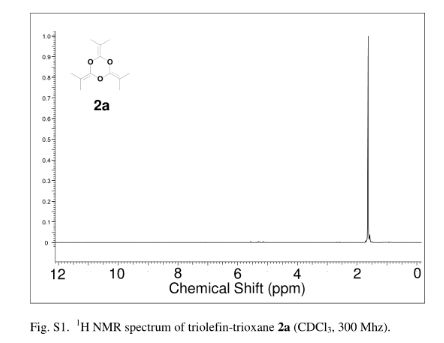
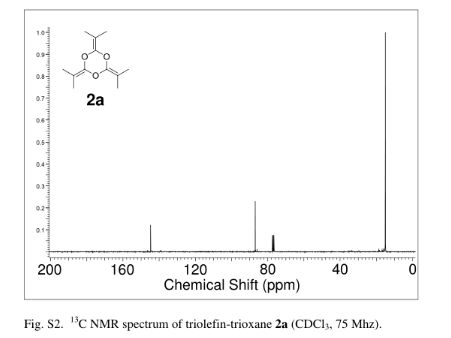
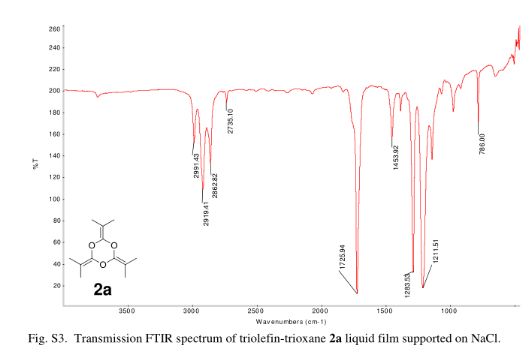
2,4,6-Tri(propan-2-ylidene)-1,3,5-trioxane (2a)
crude
product was purified by vacuum distillation (10 mmHg at 185 °C) to
yield the title compound as a colorless liquid (2.32 g, 71%). 1H NMR (CDCl3, 300 MHz) δ = 1.63 (s, 18 H,) ppm; 13C NMR (CDCl3, 75 MHz) δ = 15.0, 86.9, 144.7 ppm; IR νmax (liquid) 2991, 2919, 2863, 1726, 1284, 1212 cm–1; UV (CH3CN) λmax = 210 nm (ε = 1.57 × 104 L/mol·cm); HRMS (ESI) m/z calcd for C12H18O3 [M + H]+ 211.1334, found 211.1342. Anal. Calcd for C12H18O3: C, 68.54; H, 8.68; O, 22.83. Found: C, 68.48; H; 8.76.
/////////Synthesis, Low Temperature, Spectroscopic Observation, of 1,3,5-Trioxane-2,4,6-Trione, The Cyclic Trimer, Carbon Dioxide
/////////////
Torkham, PAKISTAN, AFGHANISTAN , BORDER
Torkham
is one of the major border crossings between Afghanistan and Pakistan,
located on the Torkham international border. It connects Nangarhar
province of Afghanistan with Pakistan's Federally Administered Tribal
Areas and Khyber Pakhtunkhwa.
Wikipedia
Torkham
تورخم
|
|---|

Khyber Pass gate at Torkham, Pakistan
|
|
|























 //////////
//////////