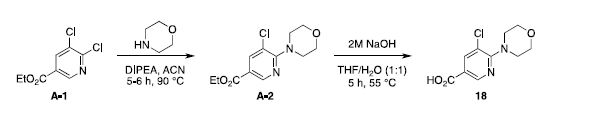
Preparation of 5-chloro-6-morpholinonicotinic acid (18)1
A solution of ethyl 5,6-dichloronicotinate (A-1, 1.1 g, 5.0 mmol), N,N-diisopropylethylamine (DIPEA, 0.97
g, 7.5 mmol) in acetonitrile (3.0 mL) was treated with morpholine (0.48 g, 6.0 mmol). The mixture was
stirred for 6 hours at 95 °C. The complete conversion of the starting material could be detected by
observing a change of the color from yellowish to deep orange. After complete conversion, the mixture was
evaporated to dryness, the residue diluted with water (20 mL), and extracted with dichloromethane (3x20
mL). The combined organic layer was dried over sodium sulfate and evaporated under reduced pressure to
yield 1.31 g (97 %) of A-2 as a red oil. The product was identified by LC/ESI-MS analysis and used for the
next synthetic step without further purification.
The ester A-2 (1.31 g, 4.83 mmol) was dissolved in a tetrahydrofurane/water mixture (1:1, 4.0 mL) and
treated with 2.0 M sodium hydroxide (0.33 g, 5.80 mmol). The reaction mixture was stirred for 5 h at 55 °C.
After complete conversion, the mixture was cooled to room temperature, diluted with water (20 mL) and
extracted with ethyl acetate (3x20 mL). The water layer was neutralized with an aqueous solution of
hydrochloric acid (2.0 N) until a white precipitate was observed. The product was collected by repeated
filtration and evaporation. The combined product fractions were dried at 70 °C yielding 1.17 g (100 %) of
18 as a white solid,
A solution of ethyl 5,6-dichloronicotinate (A-1, 1.1 g, 5.0 mmol), N,N-diisopropylethylamine (DIPEA, 0.97
g, 7.5 mmol) in acetonitrile (3.0 mL) was treated with morpholine (0.48 g, 6.0 mmol). The mixture was
stirred for 6 hours at 95 °C. The complete conversion of the starting material could be detected by
observing a change of the color from yellowish to deep orange. After complete conversion, the mixture was
evaporated to dryness, the residue diluted with water (20 mL), and extracted with dichloromethane (3x20
mL). The combined organic layer was dried over sodium sulfate and evaporated under reduced pressure to
yield 1.31 g (97 %) of A-2 as a red oil. The product was identified by LC/ESI-MS analysis and used for the
next synthetic step without further purification.
The ester A-2 (1.31 g, 4.83 mmol) was dissolved in a tetrahydrofurane/water mixture (1:1, 4.0 mL) and
treated with 2.0 M sodium hydroxide (0.33 g, 5.80 mmol). The reaction mixture was stirred for 5 h at 55 °C.
After complete conversion, the mixture was cooled to room temperature, diluted with water (20 mL) and
extracted with ethyl acetate (3x20 mL). The water layer was neutralized with an aqueous solution of
hydrochloric acid (2.0 N) until a white precipitate was observed. The product was collected by repeated
filtration and evaporation. The combined product fractions were dried at 70 °C yielding 1.17 g (100 %) of
18 as a white solid,
m.p. 185.2–186.2;
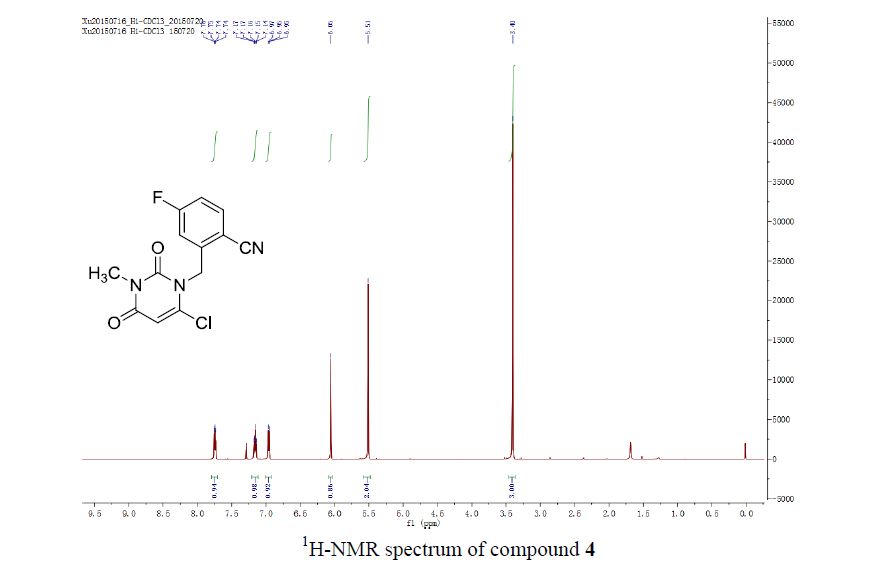
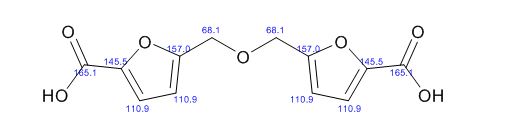
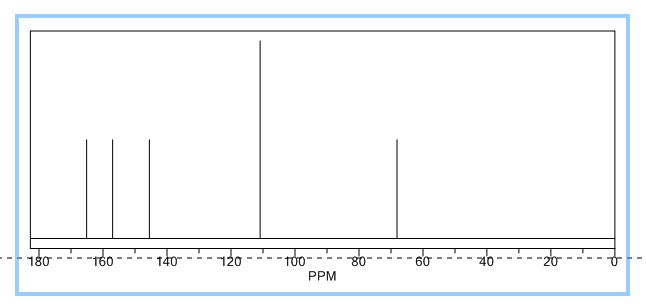
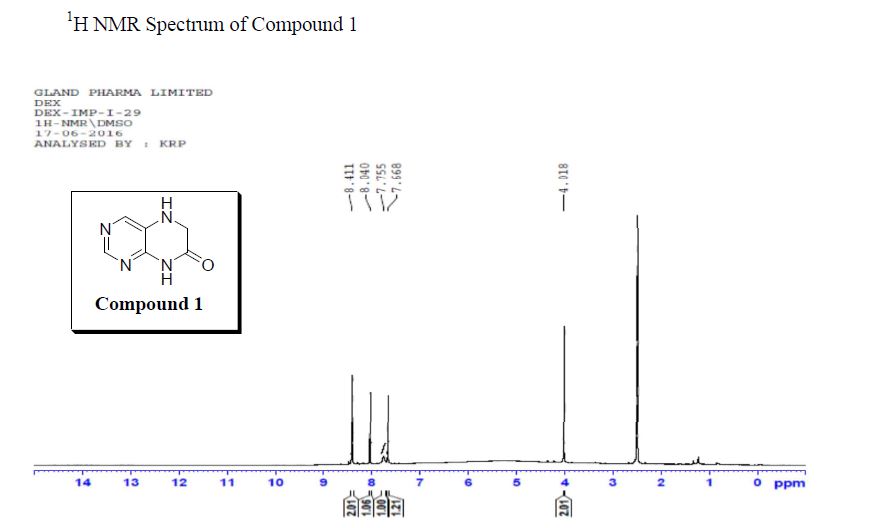
1H NMR (500 MHz, DMSO-d6) δ (ppm) 3.45 (t, J = 5.04 Hz, 2xCH2,4H), 3.72 (t, J = 4.41 Hz, 2xCH2, 4H), 8.08 (d, J = 1.89 Hz, CH, 1H), 8.66 (d, J = 1.90 Hz, CH, 1H), 13.01(s, CO2H, 1H). 13C (125 MHz, DMSO-d6) δ (ppm) 48.9 (2xCH2), 66.1 (2xCH2), 119.4, 139.7, 147.5, 159.3,
165.4;
165.4;
LC/ESI-MS (m/z): 243.28 [M+H]+; Purity: 100.0 % (N).
Colandrea, V. J.; Doherty, G. A; Hale, J. J.; Huo, P.; Legiec, I. E.; Toth, L.; Vachal, P.; Yan, L. (3,4-
Disubstituted)propanoic carboxylates as S1P (EDG) receptor agonists. PCT Int. Appl. WO2005058848A1, 2005 (Merck & Co., Inc.).
Disubstituted)propanoic carboxylates as S1P (EDG) receptor agonists. PCT Int. Appl. WO2005058848A1, 2005 (Merck & Co., Inc.).
Supporting Information
https://s3-eu-west-1.amazonaws.com/pstorage-acs-6854636/.../jm500729a_si_001.pdf
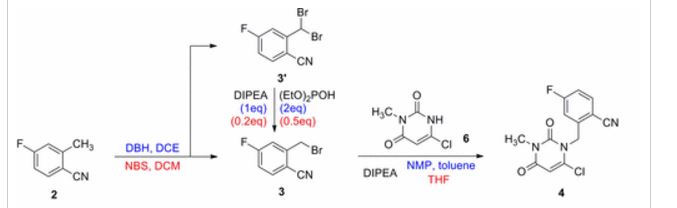
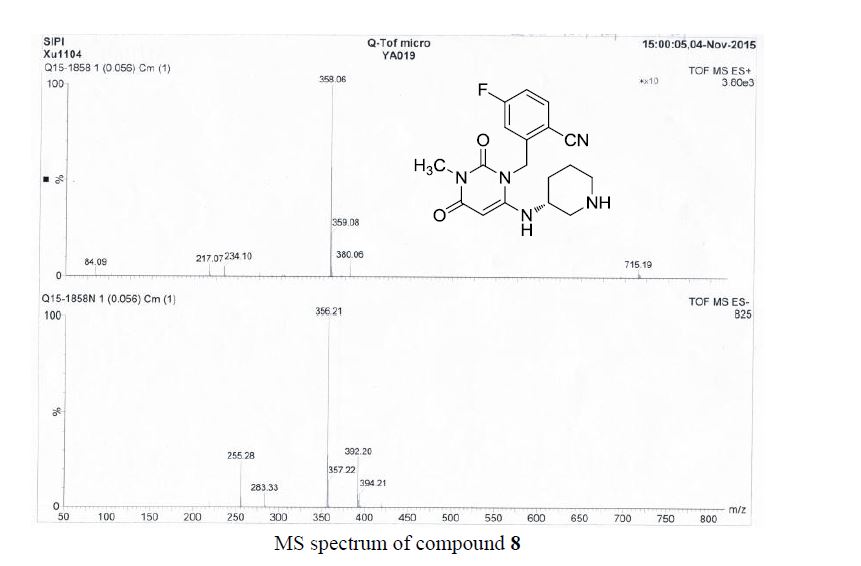

![Graphical abstract: A two-step efficient preparation of a renewable dicarboxylic acid monomer 5,5′-[oxybis(methylene)]bis[2-furancarboxylic acid] from d-fructose and its application in polyester synthesis](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C6GC03314H)
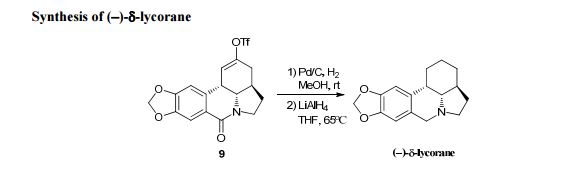
 :
: