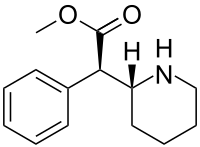
DEXMETHYLPHENIDATE
SynonymsDexmethylphenidate HCl, UNII1678OK0E08, CAS Number19262-68-1, WeightAverage: 269.77
Chemical FormulaC14H20ClNO2
methyl (2R)-2-phenyl-2-[(2R)-piperidin-2-yl]acetate hydrochloride
| CAS Number |
|
|---|---|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
CLIP
An Improved and Efficient Process for the Production of Highly Pure Dexmethylphenidate Hydrochloride
Long-Xuan Xing, Cheng-Wu Shen, Yuan-Yuan Sun, Lei Huang, Yong-Yong Zheng,* Jian-Qi Li*
https://onlinelibrary.wiley.com/doi/abs/10.1002/jhet.2705
The present work describes an efficient and commercially viable process for the synthesis of dexmethylphenidate hydrochloride (1), a mild nervous system stimulant. The overall yield is 23% with ~99.9% purity (including seven chemical steps). Formation and control of possible impurities are also described in this report.

(R)-methyl 2-phenyl-2-((R)-piperidin-2-yl)acetate hydrochloride (1). ............ afford 1 as a white solid (107.6 g, 87.3% yield) with 99.50% purity and 99.70% ee. The crude product (107.6 g, 0.4 mol) was further purified by recrystallization from pure water (100 mL) to obtain the qualified product 1 (98.3 g, 91.4% yield) with 99.92 purity and 99.98% ee.
[α] 25 D +85.6 (MeOH, c 1) (lit [4b]. [α] 25 D +84 (MeOH, c 1));
Mp 222-223 C (lit [4b]. Mp 222– 224°C); MS m/z 234 [M + H]+ .
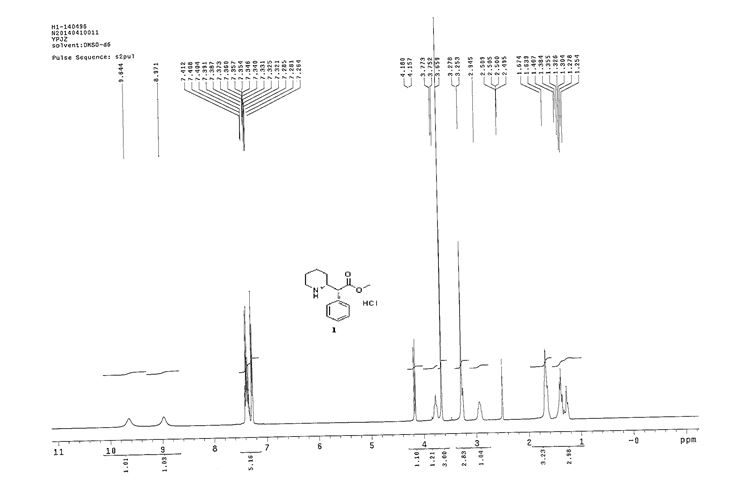
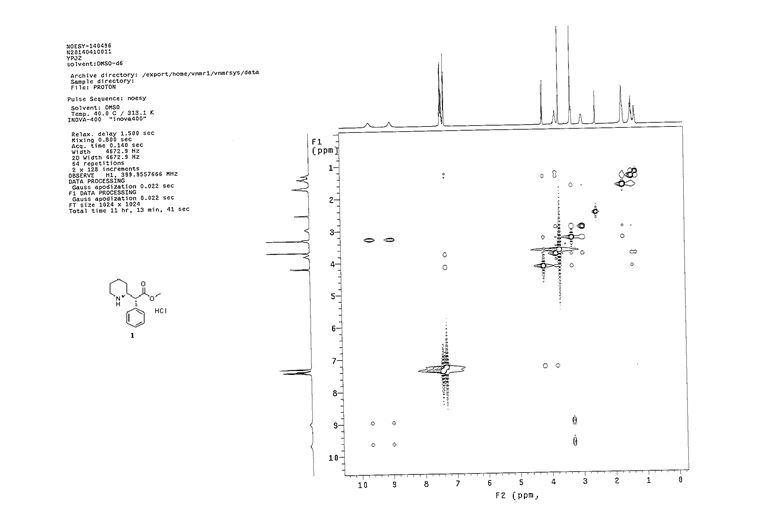
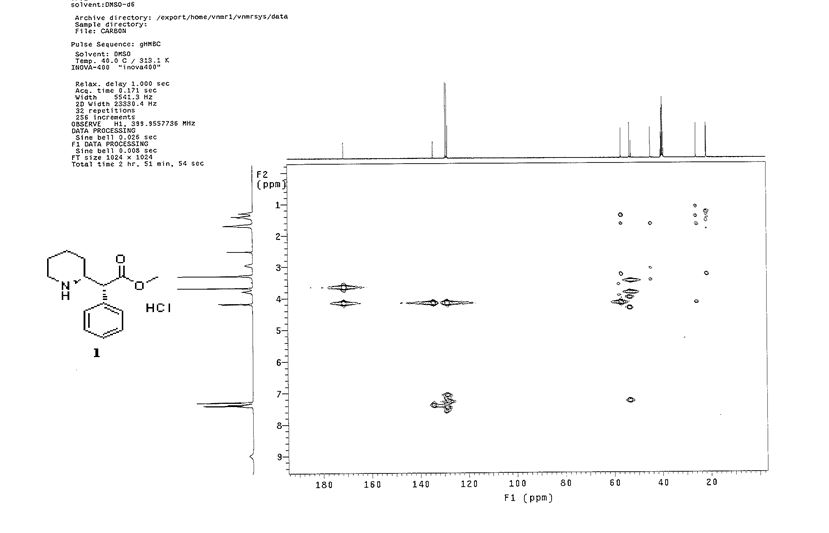
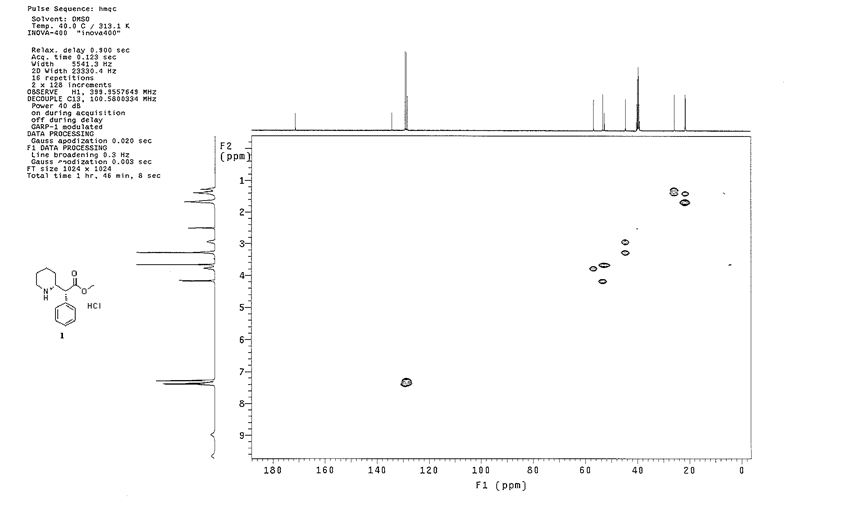
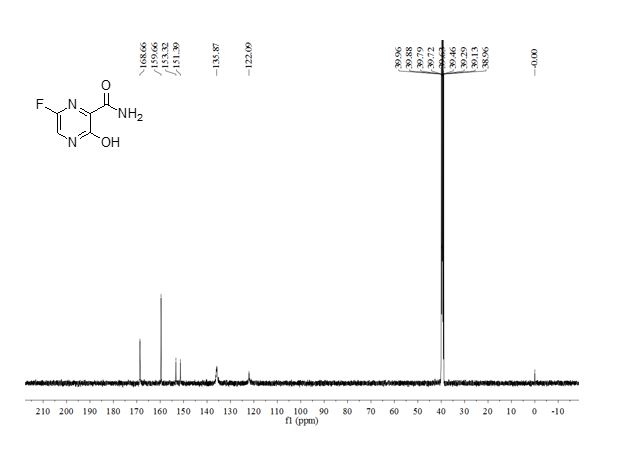
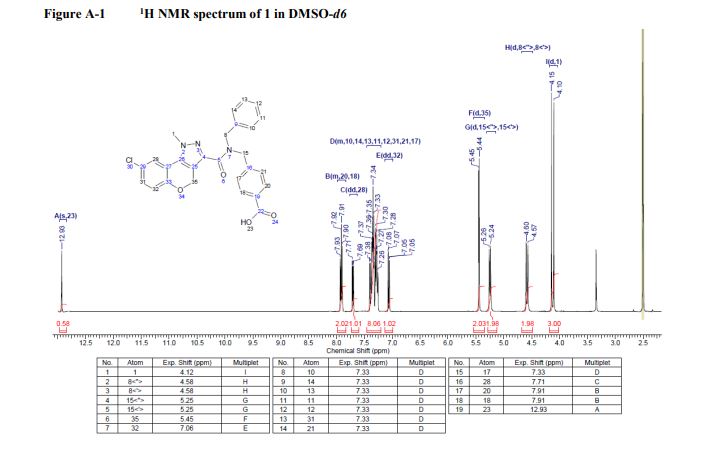
1 H NMR (400Hz, DMSO-d6) δ 1 H NMR (400 MHz, DMSO-d6) δ 9.64 (br, 1H), 8.97 (br, 1H), 7.41-7.26 (m, 5H), 4.18-4.16 (d, J = 9.2Hz, 1H), 3.77-3.75 (m, 1H), 3.66 (s, 3H), 3.25 (m, 1H), 2.94 (m, 1H), 1.67-1.64 (m, 3H), 1.41-1.25 (m, 3H).
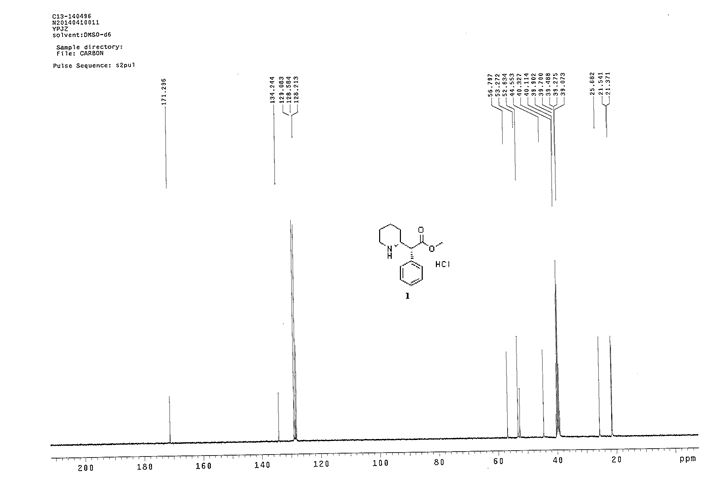
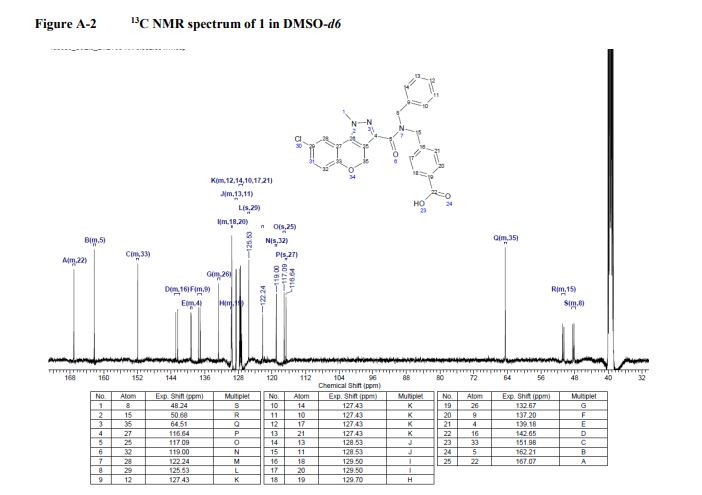
13C NMR (100.6 MHz, DMSO-d6) δ 171.3, 134.2, 129.1, 128.6, 128.2, 56.8, 53.3, 52.6, 44.5, 25.7, 21.5, 21.4.
1H-NMR, and 13C-NMR of compound 1......................................... 10-11
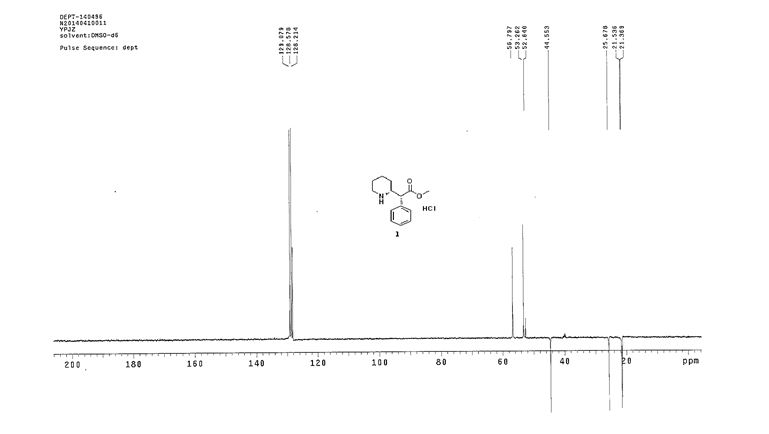
DEPT,
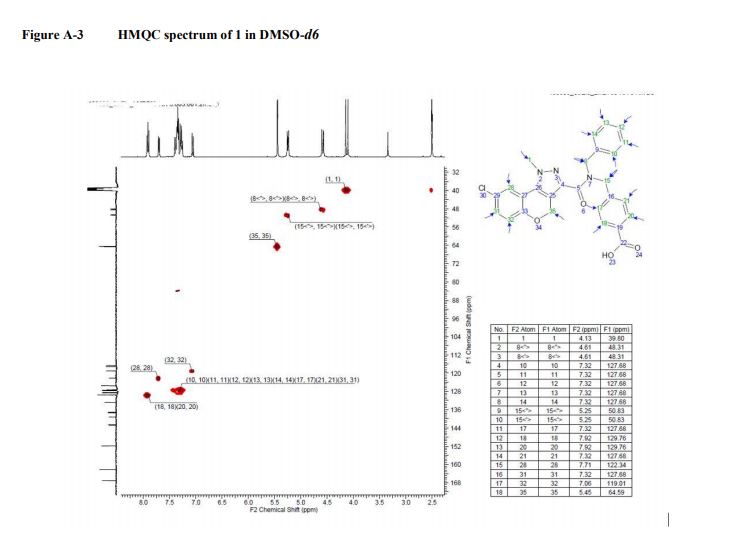
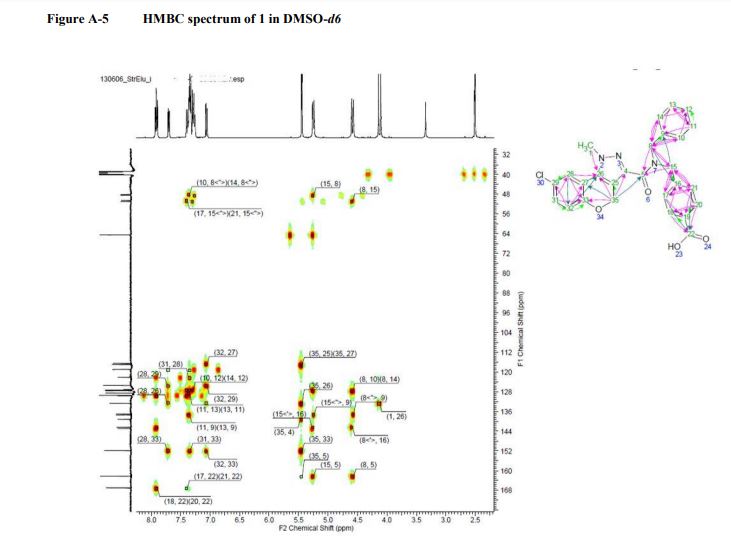
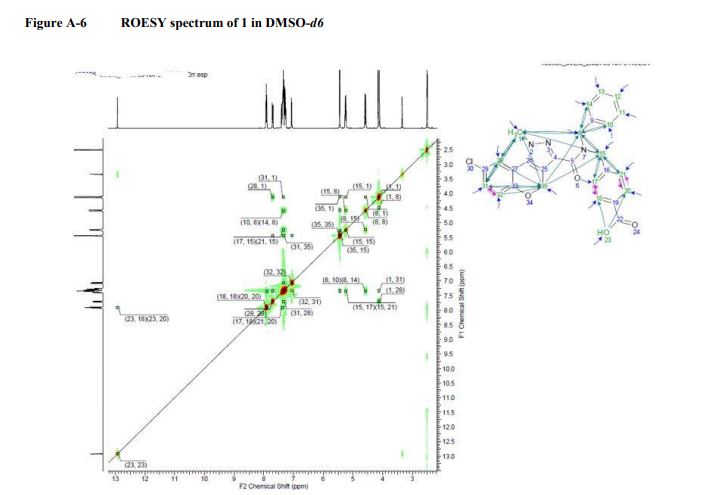
COSY, NOESY, GHMBC, and HMQC of compound 1.................. 12-14
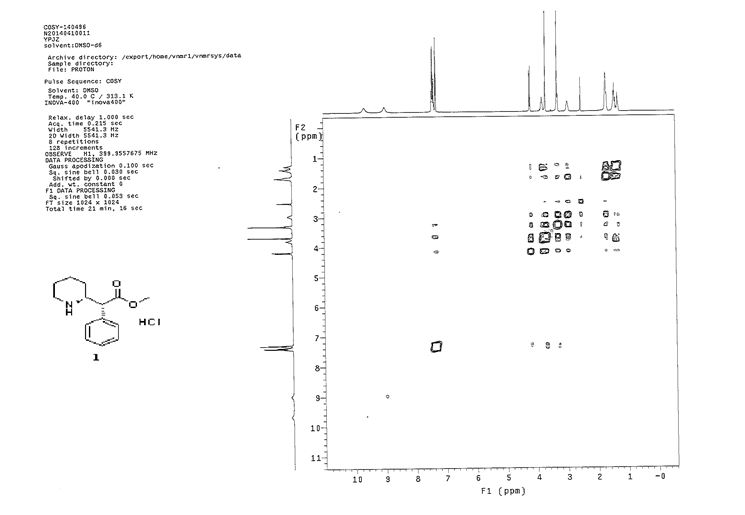
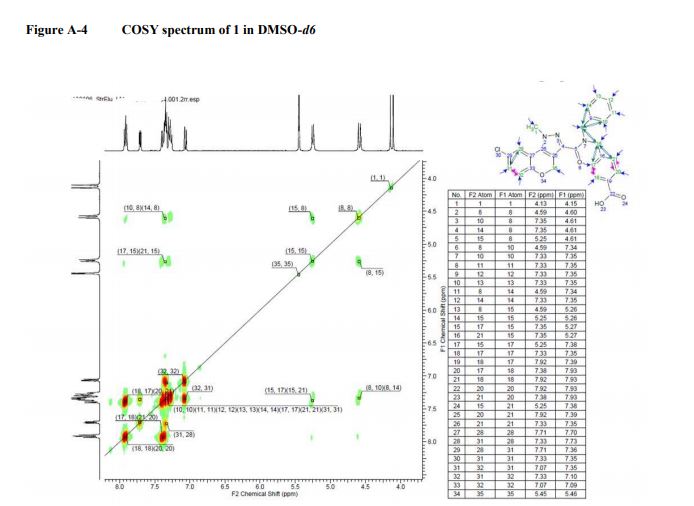
COSY
NOESY
GHMBC