
See part 2 athttp://orgspectroscopyint.blogspot.in/2016/02/haloperidol-22.html
Haloperidol /hæloʊpɛridɒl/ (INN, BAN, USAN, AAN; most common brand names: Haldol, Serenace) is an antipsychotic medication used in the treatment of schizophrenia, acute psychosis, mania, delirium, tics in Tourette syndrome, choreas, nausea and vomiting inpalliative care, intractable hiccups, agitation and severe anxiety.[3][4][5] Haloperidol is a butyrophenone derivative and functions as aninverse agonist of dopamine. It is classified as a typical antipsychotic and has pharmacological effects similar to the phenothiazines.[4]
Haloperidol /hæloʊpɛridɒl/ (INN, BAN, USAN, AAN; most common brand names: Haldol, Serenace) is an antipsychotic medication used in the treatment of schizophrenia, acute psychosis, mania, delirium, tics in Tourette syndrome, choreas, nausea and vomiting inpalliative care, intractable hiccups, agitation and severe anxiety.[3][4][5] Haloperidol is a butyrophenone derivative and functions as aninverse agonist of dopamine. It is classified as a typical antipsychotic and has pharmacological effects similar to the phenothiazines.[4]
A long-acting decanoate ester of haloperidol is used as an injection given every four weeks to people with schizophrenia or related illnesses who have poor adherence to medication regimens (most commonly due to them forgetting to take their medication, or due to poor insight into their illness) and suffer frequent relapses of illness, or to overcome the drawbacks inherent to its orally administered counterpart.[6] Such long acting injections are controversial because it can be seen as denying people their right to stop taking their medication.
It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[7]
Skeletal formula of haloperidol decanoate: The decanoate group is highlighted in blue.
Brief background information
| Salt | ATC | Formula | MM | CAS |
|---|---|---|---|---|
| - | N05AD01 | C 21 H 23 ClFNO 2 | 375.87 g / mol | 52-86-8 |
Application
- neuroleptic
- antidiskinetik
- antipsychotic
- dopamine antagonists
Classes of substances
- Chloro alcohols
- p-Ftorbutirofenony 4-piperidinyl derivatives
- Piperidinol
Synthesis pathway
Trade Names
| Country | Trade name | Manufacturer |
|---|---|---|
| Germany | Haldol-Janssen | Janssen-Cilag |
| various generic drugs | ||
| France | Haldol | Janssen-Cilag |
| United Kingdom | - "- | - "- |
| Serenak | Ivax | |
| Italy | Haldol | Janssen-Cilag |
| Serenas | Lusofarmaco | |
| Japan | Galomont | Janssen - Dainippon Sumitomo |
| Neoperidol | Janssen | |
| Serenak | Dainippon Sumitomo | |
| USA | various generic drugs | |
| Ukraine | Haloperidol | Ltd. "Gedeon Richter", Hungary |
| various generic drugs | ||
Formulations
- ampoules of 5 mg / 1 ml, 100 mg / ml, 50 mg / ml;
- drops of 2 mg to 20 mg / ml, 2 mg / ml, 0.5 mg / ml;
- garnuly 1%;
- Powder 1%;
- 0.2% solution, 10 mg;
- oral solution 2 mg / ml, 10 mg / ml;
- Tablets of 0.75 mg, 1 mg, 1.5 mg, 2 mg, 3 mg, 5 mg, 10 mg, 20 mg
Links
- Janssen, PAJ et al .: J. Med. Pharm. Chem. (JMPCAS) 1, 281 (1959).
- DE 1289845 (Janssen; appl. 18/4/1959; GB -prior. 4.22.1958).
- US 3,438,991 (Janssen; 4.15.1969; GB -prior. 18.11.1959).
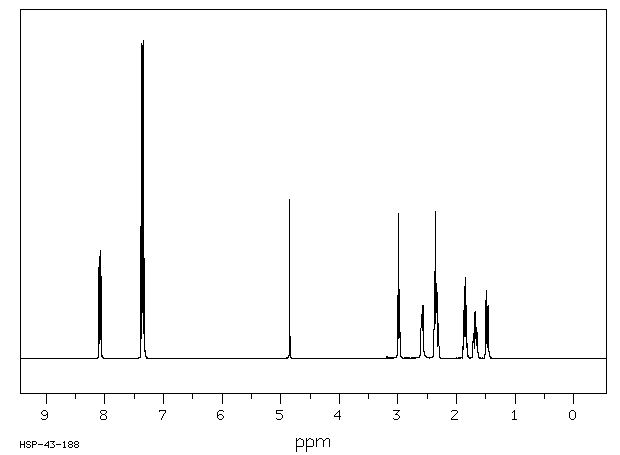
1H NMR
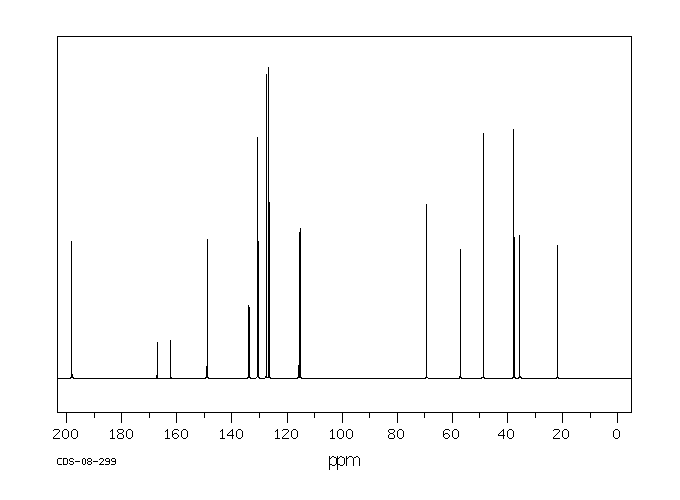
13 C NMR
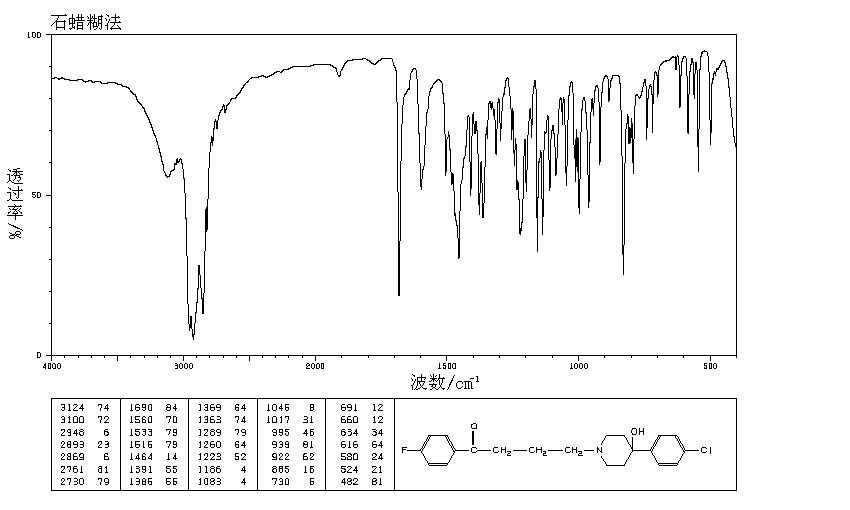
IR
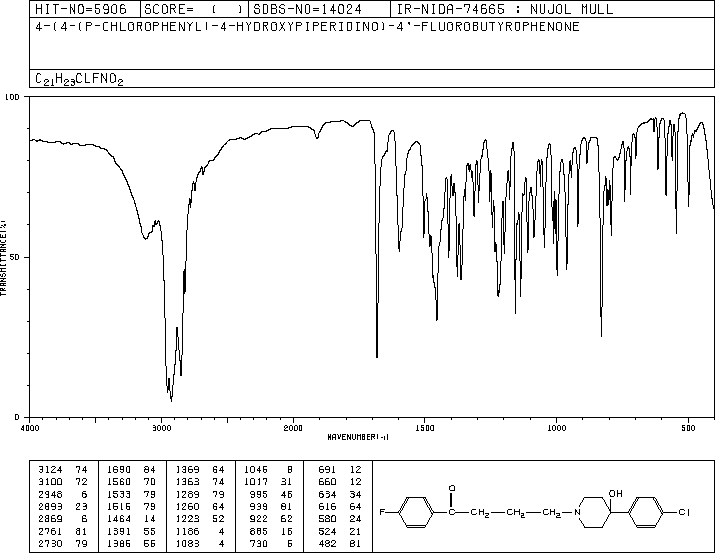
MASS

| Systematic (IUPAC) name | |
|---|---|
| 4-[4-(4-Chlorophenyl)-4-hydroxy-1-piperidyl]-1-(4-fluorophenyl)-butan-1-one | |
| Clinical data | |
| Trade names | Haldol |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682180 |
| Pregnancy cat. | C (AU) C (US) |
| Legal status | Prescription Only (S4) (AU) ℞-only (CA) POM (UK) ℞-only (US) |
| Routes | Oral, IM, IV, depot (asdecanoate ester) |
| Pharmacokinetic data | |
| Bioavailability | 60-70% (Oral)[1] |
| Protein binding | ~90%[1] |
| Metabolism | Liver-mediated[1] |
| Half-life | 14-26 hours (IV), 20.7 hours (IM), 14-37 hours (oral)[1] |
| Excretion | Biliary (hence in faeces) and in urine[1][2] |
| Identifiers | |
| CAS number | 52-86-8 |
| ATC code | N05AD01 |
| PubChem | CID 3559 |
| IUPHAR ligand | 86 |
| DrugBank | DB00502 |
| ChemSpider | 3438 |
| UNII | J6292F8L3D |
| KEGG | D00136 |
| ChEBI | CHEBI:5613 |
| ChEMBL | CHEMBL54 |
| Chemical data | |
| Formula | C21H23ClFNO2 |
| Mol. mass | 375.9 g/mol |
History
Haloperidol was discovered by Paul Janssen.[70] It was developed in 1958 at the Belgian company Janssen Pharmaceutica and submitted to the first of clinical trials in Belgiumlater that year.[71]
Haloperidol was approved by the U.S. Food and Drug Administration (FDA) on April 12, 1967; it was later marketed in the U.S. and other countries under the brand name Haldol byMcNeil Laboratories.[citation needed]
Society and culture
Coincident with civil unrest in the United States in the 1960s and 1970s, schizophrenia was racialized to match the behavior of angry/violent black men. Haldol was promoted as a way to pacify them, and was marketed to appeal to feelings of racial unease. (cf. Metzl 2010. The Protest Psychosis)
See also: Punitive psychiatry in the Soviet Union
Soviet dissidents, including medical staff, have reported several times on the use of haloperidol in the Soviet Union for punitive purposes or simply to break the prisoners' will.[72][73][74] Notable dissidents who were administered haloperidol as part of their court-ordered treatment were Sergei Kovalev and Leonid Plyushch.[75] The accounts Plyushch gave in the West, after he was allowed to leave the Soviet Union in 1976, were instrumental in triggering Western condemnation of Soviet practices at the World Psychiatric Association's 1977 meeting.[76] The use of haloperidol in the Soviet Union's psychiatric system was prevalent because it was one of the few psychotropic drugs produced in quantity in the USSR.[77]
Haloperidol has been used for its sedating effects during the deportations of immigrants by the United States Immigration and Customs Enforcement (ICE). During 2002-2008, federal immigration personnel used haloperidol to sedate 356 deportees. By 2008, following court challenges over the practice, it was given to only three detainees. Following lawsuits, U.S. officials changed the procedure so the drug is administered only by the recommendation of medical personnel and under court order.[78][79]
Brand names
Haloperidol is sold under the tradenames Aloperidin, Bioperidolo, Brotopon, Dozic, Duraperidol (Germany), Einalon S, Eukystol, Haldol (common tradename in the US and UK), Halosten, Keselan, Linton, Peluces, Serenace and Sigaperidol
Veterinary use
Haloperidol is also used on many different kinds of animals. It appears to be particularly successful when given to birds, e.g., a parrot that will otherwise continuously pluck its feathers out.[80]
References
- Kudo, S; Ishizaki T (December 1999). "Pharmacokinetics of haloperidol: an update". Clinical pharmacokinetics 37 (6): 435-456. doi:10.2165/00003088-199937060-00001. PMID 10628896.
- "PRODUCT INFORMATION Serenace" (PDF). TGA eBusiness Services. Aspen Pharma Pty Ltd. 29 September 2011. Retrieved 29 May 2014.
- Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. p. 229-230. ISBN 978-0-85711-084-8. edit
- Brayfield, A, ed. (13 December 2013). "Haloperidol". Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. Retrieved 29 May 2014.
- "TGA Approved Terminology for Medicines" (PDF). Therapeutic Goods Administration. Australian Government, Department of Health and Ageing. July 1999. p. 66. Retrieved 29 May 2014.
- Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3. edit
- "WHO Model List of Essential Medicines" (PDF). World Health Organization. October 2013. p. 7, 35. Retrieved 22 April 2014.
- "Haldol Official FDA information, side effects and uses". Drugs.com. Retrieved 2013-10-03.
- Giannini, A. James; Underwood, Ned A.; Condon, Maggie (2000). "Acute Ketamine Intoxication Treated by Haloperidol". American Journal of Therapeutics 7 (6): 389–91.doi:10.1097/00045391-200007060-00008. PMID 11304647.
- Giannini, A. James; Eighan, Michael S.; Loiselle, Robert H.; Giannini, Matthew C. (1984). "Comparison of Haloperidol and Chlorpromazine in the Treatment of Phencyclidine Psychosis". The Journal of Clinical Pharmacology 24 (4): 202–4.doi:10.1002/j.1552-4604.1984.tb01831.x. PMID 6725621.
- Cavanaugh, SV (1986). "Psychiatric emergencies". The Medical clinics of North America 70 (5): 1185–202. PMID 3736271.
- Currier, Glenn W. (2003). "The Controversy over 'Chemical Restraint' In Acute Care Psychiatry". Journal of Psychiatric Practice 9 (1): 59–70. doi:10.1097/00131746-200301000-00006. PMID 15985915.
- Irving, Claire B; Adams, Clive E; Lawrie, Stephen (2006). "Haloperidol versus placebo for schizophrenia". In Irving, Claire B. Cochrane Database of Systematic Reviews (4): CD003082. doi:10.1002/14651858.CD003082.pub2. PMID 17054159.
- Allen, MH; Currier, GW; Hughes, DH; Reyes-Harde, M; Docherty, JP; Expert Consensus Panel for Behavioral Emergencies (2001). "The Expert Consensus Guideline Series. Treatment of behavioral emergencies". Postgraduate Medicine (Spec No): 1–88; quiz 89–90. PMID 11500996.
- Allen, Michael H.; Currier, Glenn W.; Hughes, Douglas H.; Docherty, John P.; Carpenter, Daniel; Ross, Ruth (2003). "Treatment of Behavioral Emergencies: A Summary of the Expert Consensus Guidelines". Journal of Psychiatric Practice 9 (1): 16–38. doi:10.1097/00131746-200301000-00004. PMID 15985913.
- Allen, Michael H.; Currier, Glenn W.; Carpenter, Daniel; Ross, Ruth W.; Docherty, John P. (2005). "Introduction: Methods, Commentary, and Summary". Journal of Psychiatric Practice 11: 5. doi:10.1097/00131746-200511001-00002.
- Ballard, Clive; Lana, Marisa Margallo; Theodoulou, Megan; Douglas, Simon; McShane, Rupert; Jacoby, Robin; Kossakowski, Katja; Yu, Ly-Mee; Juszczak, Edmund; on behalf of the Investigators DART AD (2008). "A Randomised, Blinded, Placebo-Controlled Trial in Dementia Patients Continuing or Stopping Neuroleptics (The DART-AD Trial)". In Brayne, Carol. PLoS Medicine 5 (4): e76. doi:10.1371/journal.pmed.0050076.PMC 2276521. PMID 18384230. Lay summary – BBC News (April 1, 2008). "Neuroleptics provided no benefit for patients with mild behavioural problems, but were associated with a marked deterioration in verbal skills"
- "Haldol Official FDA information, side effects and uses". Drugs.com. Retrieved 2013-10-03.
- "Haloperidol at Chemeurope".
- ^ Jump up to:a b Work Group on Schizophrenia. "Practice Guideline for the Treatment of Patients With Schizophrenia Second Edition". Retrieved 21 April 2014.
- Oosthuizen, P.; Emsley, R. A.; Turner, J.; Keyter, N. (2001). "Determining the optimal dose of haloperidol in first-episode psychosis". Journal of Psychopharmacology 15 (4): 251–5. doi:10.1177/026988110101500403. PMID 11769818.
- Tauscher, Johannes; Kapur, Shitij (2001). "Choosing the Right Dose of Antipsychotics in Schizophrenia". CNS Drugs 15 (9): 671–8. doi:10.2165/00023210-200115090-00001.PMID 11580306.
- Goodman and Gilman's Pharmacological Basis of Therapeutics, 10th edition (McGraw-Hill, 2001).[page needed]
- American Academy of Hospice and Palliative Medicine. "Five Things Physicians and Patients Should Question". Choosing Wisely: an initiative of the ABIM Foundation(American Academy of Hospice and Palliative Medicine). Retrieved August 1, 2013., which cites
- Smith, Thomas J.; Ritter, Joseph K.; Poklis, Justin L.; Fletcher, Devon; Coyne, Patrick J.; Dodson, Patricia; Parker, Gwendolyn (2012). "ABH Gel is Not Absorbed from the Skin of Normal Volunteers". Journal of Pain and Symptom Management 43(5): 961–6. doi:10.1016/j.jpainsymman.2011.05.017. PMID 22560361.
- Weschules, Douglas J. (2005). "Tolerability of the Compound ABHR in Hospice Patients". Journal of Palliative Medicine 8 (6): 1135–43.doi:10.1089/jpm.2005.8.1135. PMID 16351526.
- PRODUCT INFORMATION [Internet]. 2011 [cited 2013 Sep 29]. Available from:https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2011-PI-03532-3
- HALDOL® Injection FOR INTRAMUSCULAR INJECTION ONLY PRODUCT INFORMATION [Internet]. Janssen; 2011 [cited 2013 Sep 29]. Available from:https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2009-PI-00998-3
- Truven Health Analytics, Inc. DrugPoint® System (Internet) [cited 2013 Sep 29]. Greenwood Village, CO: Thomsen Healthcare; 2013.
- Joint Formulary Committee. British National Formulary (BNF) 65. Pharmaceutical Pr; 2013.
- Leucht, Stefan; Cipriani, Andrea; Spineli, Loukia; Mavridis, Dimitris; Örey, Deniz; Richter, Franziska; Samara, Myrto; Barbui, Corrado; Engel, Rolf R; Geddes, John R; Kissling, Werner; Stapf, Marko Paul; Lässig, Bettina; Salanti, Georgia; Davis, John M (2013). "Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis". The Lancet 382 (9896): 951–62.doi:10.1016/S0140-6736(13)60733-3. PMID 23810019.
- Silvestri, Simone; Seeman, Mary V.; Negrete, Juan-Carlos; Houle, Sylvain; Shammi, C.M.; Remington, Garry J.; Kapur, Shitij; Zipursky, Robert B.; Wilson, Alan A.; Christensen, Bruce K.; Seeman, Philip (2000). "Increased dopamine D 2 receptor binding after long-term treatment with antipsychotics in humans: A clinical PET study".Psychopharmacology 152 (2): 174–80. doi:10.1007/s002130000532.PMID 11057521.
- Dorph-Petersen, Karl-Anton; Pierri, Joseph N; Perel, James M; Sun, Zhuoxin; Sampson, Allan R; Lewis, David A (2005). "The Influence of Chronic Exposure to Antipsychotic Medications on Brain Size before and after Tissue Fixation: A Comparison of Haloperidol and Olanzapine in Macaque Monkeys". Neuropsychopharmacology 30 (9): 1649–61. doi:10.1038/sj.npp.1300710. PMID 15756305.
- Jump up^ Konopaske, Glenn T.; Dorph-Petersen, Karl-Anton; Sweet, Robert A.; Pierri, Joseph N.; Zhang, Wei; Sampson, Allan R.; Lewis, David A. (2008). "Effect of Chronic Antipsychotic Exposure on Astrocyte and Oligodendrocyte Numbers in Macaque Monkeys". Biological Psychiatry 63 (8): 759–65.doi:10.1016/j.biopsych.2007.08.018. PMC 2386415. PMID 17945195.
- Jump up^ Vernon, Anthony C.; Natesan, Sridhar; Modo, Mike; Kapur, Shitij (2011). "Effect of Chronic Antipsychotic Treatment on Brain Structure: A Serial Magnetic Resonance Imaging Study with Ex Vivo and Postmortem Confirmation". Biological Psychiatry 69 (10): 936–44. doi:10.1016/j.biopsych.2010.11.010. PMID 21195390.
- Jump up^ Breggin, Peter R. (2007). "Neuroleptic-Induced Neurotoxicity, Brain Damage, Persistent Cognitive Deficits, Dementia, and Psychosis". Brain disabling treatments in psychiatry. Springer. pp. 85–114. ISBN 0-8261-2934-X.
- Jump up^ Halbreich, U; Shen, J; Panaro, V (1996). "Are chronic psychiatric patients at increased risk for developing breast cancer?". The American Journal of Psychiatry 153 (4): 559–60. PMID 8599407.
- Jump up^ Dalton, Susanne Oksbjerg; Mellemkjaer, Lene; Thomassen, Lars; Mortensen, Preben B.; Johansen, Christoffer (2005). "Risk for cancer in a cohort of patients hospitalized for schizophrenia in Denmark, 1969–1993". Schizophrenia Research 75 (2–3): 315–24.doi:10.1016/j.schres.2004.11.009. PMID 15885523.
- Jump up^ Grinshpoon, Alexander; Barchana, Micha; Ponizovsky, Alexander; Lipshitz, Irena; Nahon, Daniella; Tal, Orna; Weizman, Abraham; Levav, Itzhak (2005). "Cancer in schizophrenia: Is the risk higher or lower?". Schizophrenia Research 73 (2–3): 333–41.doi:10.1016/j.schres.2004.06.016. PMID 15653279.
- Jump up^ Szarfman, Ana; Tonning, Joseph M; Levine, Jonathan G; Doraiswamy, P. Murali (2006). "Atypical Antipsychotics and Pituitary Tumors: A Pharmacovigilance Study".Pharmacotherapy 26 (6): 748–58. doi:10.1592/phco.26.6.748. PMID 16716128.
- Jump up^ Hippisley-Cox, Julia; Vinogradova, Y; Coupland, C; Parker, C (2007). "Risk of Malignancy in Patients with Schizophrenia or Bipolar Disorder". Archives of General Psychiatry 64 (12): 1368–76. doi:10.1001/archpsyc.64.12.1368. PMID 18056544.
- Jump up^ Levav, Itzhak; Kohn, Robert; Barchana, Micha; Lipshitz, Irena; Pugachova, Inna; Weizman, Abraham; Grinshpoon, Alexander (2009). "The risk for cancer among patients with schizoaffective disorders". Journal of Affective Disorders 114 (1–3): 316–20.doi:10.1016/j.jad.2008.06.010. PMID 18675461.
- Jump up^ De Hert, M; Correll, CU; Bobes, J; Cetkovich-Bakmas, M; Cohen, D; Asai, I; Detraux, J; Gautam, S; Möller, HJ; Ndetei, DM; Newcomer, JW; Uwakwe, R; Leucht, S (2011)."Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care". World psychiatry 10 (1): 52–77.PMC 3048500. PMID 21379357.
- Jump up^ Leentjens, Albert FG; Van Der Mast, Rose C (2005). "Delirium in elderly people: An update". Current Opinion in Psychiatry 18 (3): 325–30.doi:10.1097/01.yco.0000165603.36671.97. PMID 16639157.
- Jump up^ Sandyk, R; Hurwitz, MD (1983). "Toxic irreversible encephalopathy induced by lithium carbonate and haloperidol. A report of 2 cases". South African medical journal 64 (22): 875–6. PMID 6415823.
- Jump up^ Bush, S. E.; Hatton, R. C.; Winterstein, A. G.; Thomson, M. R.; Woo, G. W. (2008). "Effects of concomitant amiodarone and haloperidol on Q-Tc interval prolongation".American Journal of Health-System Pharmacy 65 (23): 2232–6.doi:10.2146/ajhp080039. PMID 19020191.
- Jump up^ Igarashi, K.; Kasuya, F.; Fukui, M.; Usuki, E.; Castagnoli Jr, N. (1995). "Studies on the metabolism of haloperidol (HP): The role of CYP3A in the production of the neurotoxic pyridinium metabolite HPP+ found in rat brain following ip administration of HP". Life Sciences 57 (26): 2439–46. doi:10.1016/0024-3205(95)02240-5. PMID 8847965.
- Jump up^ Usuki, Etsuko; Pearce, Robin; Parkinson, Andrew; Castagnoli, Neal (1996). "Studies on the Conversion of Haloperidol and Its Tetrahydropyridine Dehydration Product to Potentially Neurotoxic Pyridinium Metabolites by Human Liver Microsomes". Chemical Research in Toxicology 9 (4): 800–6. doi:10.1021/tx960001y. PMID 8831826.
- Jump up^ Avent, Kathryn M.; Devoss, J. J.; Gillam, Elizabeth M. J. (2006). "Cytochrome P450-Mediated Metabolism of Haloperidol and Reduced Haloperidol to Pyridinium Metabolites".Chemical Research in Toxicology 19 (7): 914–20. doi:10.1021/tx0600090.PMID 16841959.
- Jump up^ Avent, Kathryn M.; Riker, Richard R.; Fraser, Gilles L.; Van Der Schyf, Cornelis J.; Usuki, Etsuko; Pond, Susan M. (1997). "Metabolism of haloperidol to pyridinium species in patients receiving high doses intravenously: Is HPTP an intermediate?". Life Sciences61 (24): 2383–90. doi:10.1016/S0024-3205(97)00955-7. PMID 9399630.
- Jump up^ Kawashima, Hidekazu; Iida, Yasuhiko; Kitamura, Youji; Saji, Hideo (2004). "Binding of 4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]pyridinium ion (HPP+), a metabolite of haloperidol, to synthetic melanin: Implications for the dopaminergic neurotoxicity of HPP+". Neurotoxicity Research 6 (7–8): 535–42. doi:10.1007/BF03033449.PMID 15639785.
- ^ Jump up to:a b Rollema, H; Skolnik, M; d'Engelbronner, J; Igarashi, K; Usuki, E; Castagnoli Jr, N (1994). "MPP(+)-like neurotoxicity of a pyridinium metabolite derived from haloperidol: In vivo microdialysis and in vitro mitochondrial studies". The Journal of Pharmacology and Experimental Therapeutics 268 (1): 380–7. PMID 8301579.
- Jump up^ Mythri, Rajeswara Babu; Jagatha, Balusamy; Pradhan, Nityananda; Andersen, Julie; Bharath, M. M. Srinivas (2006). "Mitochondrial Complex I Inhibition in Parkinson's Disease: How Can Curcumin Protect Mitochondria?". Antioxidants & Redox Signaling 9(3): 399–408. doi:10.1089/ars.2007.9.ft-25. PMID 17184173.
- Jump up^ Beal, M. Flint (2006). "Mitochondria, Oxidative Damage, and Inflammation in Parkinson's Disease". Annals of the New York Academy of Sciences 991: 120–31.doi:10.1111/j.1749-6632.2003.tb07470.x. PMID 12846981.
- Jump up^ Bishnoi, Mahendra; Chopra, Kanwaljit; Kulkarni, Shrinivas K. (2008). "Activation of striatal inflammatory mediators and caspase-3 is central to haloperidol-induced orofacial dyskinesia". European Journal of Pharmacology 590 (1–3): 241–5.doi:10.1016/j.ejphar.2008.06.033. PMID 18590723.
- Jump up^ Eyles, Darryl W.; Avent, Kathryn M.; Stedman, Terry J.; Pond, Susan M. (1997). "Two pyridinium metabolites of haloperidol are present in the brain of patients at post-mortem".Life Sciences 60 (8): 529–34. doi:10.1016/S0024-3205(96)00656-X. PMID 9042387.
- Jump up^ Ulrich, Sven; Neuhof, Sabine; Braun, Verena; Danos, Peter; Pester, Uwe; Hoy, Ludwig (2000). "Disposition of Haloperidol Pyridinium and Reduced Haloperidol Pyridinium in Schizophrenic Patients: No Relationship with Clinical Variables During Short-Term Treatment". Journal of Clinical Psychopharmacology 20 (2): 210–9.doi:10.1097/00004714-200004000-00014. PMID 10770460.
- Ulrich, S.; Sandmann, U.; Genz, A. (2005). "Serum Concentrations of Haloperidol Pyridinium Metabolites and the Relationship with Tardive Dyskinesia and Parkinsonism: A Cross-Section Study in Psychiatric Patients". Pharmacopsychiatry 38 (4): 171–7.doi:10.1055/s-2005-871240. PMID 16025420.
- "Haloperidol at Drugs.com".
- Seeman, P; Tallerico, T (1998). "Antipsychotic drugs which elicit little or no Parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors". Molecular Psychiatry 3 (2): 123–34.doi:10.1038/sj.mp.4000336. PMID 9577836.
- Schotte, A; Janssen PF; Megens AA; Leysen JE (1993). "Occupancy of central neurotransmitter receptors by risperidone, clozapine and haloperidol, measured ex vivo by quantitative autoradiography". Brain Research 631 (2): 191–202. doi:10.1016/0006-8993(93)91535-z. PMID 7510574. Retrieved 21 April 2014.
- Leysen, JE; Janssen, PM; Gommeren, W; Wynants, J; Pauwels, PJ; Janssen, PA (1992). "In vitro and in vivo receptor binding and effects on monoamine turnover in rat brain regions of the novel antipsychotics risperidone and ocaperidone". Molecular Pharmacology 41 (3): 494–508. PMID 1372084.
- Malmberg, Åsa; Mikaels, Åsa; Mohell, Nina (1998). "Agonist and Inverse Agonist Activity at the Dopamine D3 Receptor Measured by Guanosine 5′-[γ-Thio]Triphosphate-[35S] Binding". The Journal of Pharmacology and Experimental Therapeutics 285 (1): 119–26. PMID 9536001.
- Leysen, JE; Janssen, PM; Megens, AA; Schotte, A (1994). "Risperidone: A novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity". The Journal of Clinical Psychiatry 55 (Suppl): 5–12.PMID 7520908.
- Cobos, Enrique J.; Del Pozo, Esperanza; Baeyens, José M. (2007). "Irreversible blockade of sigma-1 receptors by haloperidol and its metabolites in guinea pig brain and SH-SY5Y human neuroblastoma cells". Journal of Neurochemistry 102 (3): 812–25.doi:10.1111/j.1471-4159.2007.04533.x. PMID 17419803.
- Colabufo, Nicolaantonio; Berardi, Francesco; Contino, Marialessandra; Niso, Mauro; Abate, Carmen; Perrone, Roberto; Tortorella, Vincenzo (2004). "Antiproliferative and cytotoxic effects of some σ2 agonists and σ1 antagonists in tumour cell lines". Naunyn-Schmiedeberg's Archives of Pharmacology 370 (2): 106–13. doi:10.1007/s00210-004-0961-2. PMID 15322732.
- Kroeze, Wesley K; Hufeisen, Sandra J; Popadak, Beth A; Renock, Sean M; Steinberg, Seanna; Ernsberger, Paul; Jayathilake, Karu; Meltzer, Herbert Y; Roth, Bryan L (2003). "H1-Histamine Receptor Affinity Predicts Short-Term Weight Gain for Typical and Atypical Antipsychotic Drugs". Neuropsychopharmacology 28 (3): 519–26.doi:10.1038/sj.npp.1300027. PMID 12629531.
- Ilyin, VI; Whittemore, ER; Guastella, J; Weber, E; Woodward, RM (1996). "Subtype-selective inhibition of N-methyl-D-aspartate receptors by haloperidol". Molecular Pharmacology 50 (6): 1541–50. PMID 8967976.
- "drugs.com".
- Kornhuber, Johannes; Schultz, Andreas; Wiltfang, Jens; Meineke, Ingolf; Gleiter, Christoph H.; Zöchling, Robert; Boissl, Karl-Werner; Leblhuber, Friedrich; Riederer, Peter (1999). "Persistence of Haloperidol in Human Brain Tissue". The American Journal of Psychiatry 156 (6): 885–90. PMID 10360127.
- Kornhuber, Johannes; Wiltfang, Jens; Riederer, Peter; Bleich, Stefan (2006). "Neuroleptic drugs in the human brain: Clinical impact of persistence and region-specific distribution". European Archives of Psychiatry and Clinical Neuroscience 256 (5): 274–80. doi:10.1007/s00406-006-0661-7. PMID 16788768.
- Healy, David (1996). The psychopharmacologists 1. London: Chapman and Hall.ISBN 978-1-86036-008-4.[page needed]
- Granger, Bernard; Albu, Simona (2005). "The Haloperidol Story". Annals of Clinical Psychiatry 17 (3): 137–40. doi:10.1080/10401230591002048. PMID 16433054.
- Podrabinek, Aleksandr (1980). Punitive Medicine. Ann Arbor Mich.: Karoma Publishers. pp. 15–20. ISBN 0-89720-022-5.
- Kosserev, I.; Crawshaw, R. (1994). "Medicine and the Gulag". BMJ 309 (6970): 1726–30. doi:10.1136/bmj.309.6970.1726. PMC 2542687. PMID 7820004.
- de Boer, S. P.; E. J. Driessen; H. L. Verhaar (1982). Biographical Dictionary of Dissidents in the Soviet Union, 1956-1975. The Hague: Martinus Nijhoff Publishers.ISBN 90-247-2538-0.[page needed]
- Wade, N. (1976). "Sergei Kovalev: Biologist Denied Due Process and Medical Care".Science 194 (4265): 585–7. doi:10.1126/science.194.4265.585. PMID 17818411.
- "Censuring the Soviets". TIME (CNN). 1977-09-12. Retrieved 2009-06-21.
- The Children of Pavlov, TIME, Jun. 23, 1980
- "Fewer US deportees being sedated for removal". Epilepsy.com. Associated Press. 2008-12-30. Retrieved 2009-06-21.
- Solis, Dianne (2009-01-05). "U.S. cuts back on sedating deportees with Haldol". Seattle Times. Retrieved 2009-06-21.
- "Veterinary:Avian at Lloyd Center Pharmacy".
External links
- Rx-List.com - Haloperidol
- Medline plus - Haloperidol
- Swiss scientific information on Haldol
- "WHO Model List of Essential Medicines" (PDF) (16th list (updated) ed.). World Health Organization. March 2010. Retrieved 2010-09-14.
- U.S. National Library of Medicine: Drug Information Portal - Haloperidol
see part 2 at
Haloperidol
CAS Registry Number: 52-86-8
CAS Name: 4-[4-(4-Chlorophenyl)-4-hydroxy-1-piperidinyl]-1-(4-fluorophenyl)-1-butanone
Additional Names: 4-[4-(p-chlorophenyl)-4-hydroxypiperidino]-4¢-fluorobutyrophenone; 4¢-fluoro-4-(4-hydroxy-4-p-chlorophenylpiperidino)butyrophenone; 1-(3-p-fluorobenzoylpropyl)-4-p-chlorophenyl-4-hydroxypiperidine
Manufacturers' Codes: R-1625
Trademarks:
Aloperidin; Bioperidolo (Firma); Brotopon (Pfizer Taito); Dozic
(Rosemont); Einalon S (Kodama); Eukystol (Merckle); Haldol (McNeil);
Halosten (Shionogi); Keselan (Sumitomo); Linton (Yoshitomi); Peluces
(Isei); Serenace (Searle); Serenase (Lusofarmaco); Sigaperidol
(Dumex)
Molecular Formula: C21H23ClFNO2
Molecular Weight: 375.86
Percent Composition: C 67.11%, H 6.17%, Cl 9.43%, F 5.05%, N 3.73%, O 8.51%
Literature References: Prepn: P. A. J. Janssen et al., J. Med. Pharm. Chem. 1, 281 (1959); P. A. J. Janssen, BE 577977 (1959), C.A. 54, 4630c (1960); idem, GB 895309; idem, US 3438991 (1962, 1969 both to Janssen). Toxicity: E. I. Goldenthal, Toxicol. Appl. Pharmacol. 18, 185 (1971); A. J. Collins, M. Horlington, Br. J. Pharmacol. 37, 140 (1969). Metabolism in man: A. Forsman et al., Curr. Ther. Res. 21, 606 (1977). Comprehensive description: C. A. Janicki, C. Y. Ko, Anal. Profiles Drug Subs. 9, 341-369 (1980). Review of pharmacology and therapeutic efficacy of decanoate in psychosis: R. Beresford, A. Ward, Drugs 33, 31-49 (1987).
Properties: Crystals, mp 148.0-149.4°. uv max (9:1 0.1M HCl:methanol): 247, 221 nm (e 13300, 15000). pKa 8.3. Soly in water: 1.4 mg/100 ml. Freely sol in chloroform, methanol, acetone, benzene, dil acids. LD50 orally in rats: 165 mg/kg (Goldenthal); i.p. in mice: 60 mg/kg (Collins, Horlington).
Melting point: mp 148.0-149.4°
pKa: pKa 8.3
Absorption maximum: uv max (9:1 0.1M HCl:methanol): 247, 221 nm (e 13300, 15000)
Toxicity data: LD50 orally in rats: 165 mg/kg (Goldenthal); i.p. in mice: 60 mg/kg (Collins, Horlington)
Derivative Type: Hydrochloride
CAS Registry Number: 1511-16-6
Molecular Formula: C21H23ClFNO2.HCl
Molecular Weight: 412.33
Percent Composition: C 61.17%, H 5.87%, Cl 17.20%, F 4.61%, N 3.40%, O 7.76%
Properties: Crystals, mp 226-227.5°. Soly in water: 300 mg/100 ml.
Melting point: mp 226-227.5°
Derivative Type: Decanoate
CAS Registry Number: 74050-97-8
Manufacturers' Codes: KD-136
Trademarks: Haldol Decanoate (Janssen); Halomonth (Dainippon); Neoperidole (Kyowa Hakko)
Molecular Formula: C31H41ClFNO3
Molecular Weight: 530.11
Percent Composition: C 70.24%, H 7.80%, Cl 6.69%, F 3.58%, N 2.64%, O 9.05%
Therap-Cat: Antidyskinetic (in Gilles de la Tourette's disease); antipsychotic.
Keywords: Antidyskinetic; Antipsychotic; Butyrophenones.
Location of Khajuraho Group of Monuments in India.

Hotel Chandela - A Taj Leisure Hotel
|




No comments:
Post a Comment