HNMR peaks Piperonal.
As I assigned number 1-5, i have identified 5 different types of hydrogens that are going to show the five different peaks on the HNMR spectrum. Hydrogens labeled as number are equivalent so there are no coupling and are going to show a singlet but with the height of 2H. (S,2H).
Hydrogen labelled as 2 is going to show a doublet (d,1H) because of spin-spin splitting with Hydrogen 3 on the same aromatic ring. Hydrogen 2 and 3 are adjacent to each other which makes them have the spin-spin splitting. the J constant for this Hydrogen is about 8Hz which we can calculate by the difference of the ranges in PPM an multiplying by 300 .
and after the calculation i have found it to be about 7.2 which is close to 8 is valid for out argument. Hydrogen 3 show two spin-spin splitting because it it adjacent to Hydrogen 2 and meta relation to Hydrogen 5 on the aromatic ring. so Hydrogen 3 has (d,d,1H) two doublets next to each other. Hydrogen 4 is an aldehyde and had no coupling effect from the aromatic ring and therefore shows only a singlet (S,1H). also we know it is going to lie between the ranges of 9-10 because it's an aldehyde.
As i mentioned before Hydrogen 5 is experiencing meta coupling effect because of Hydrogen 3 on the aromatic ring so it is going to show a doublet (d,1H). Hydrogens 2,3,and 5 all lie between the ranges of about 7-8. Also Hydrogen 5 is going to have a J constant of about 2Hz. After the integration we see that our assumptions are correct and the length of the integration lines correlates to the numbers of hydrogen.
IR
Piperonal (1,3-benzenodioxole-5-carbaldehyde)
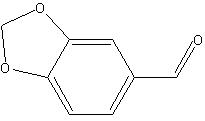
The C=O double bond can be found in the range from 165 to 210 ppm. The C=O bond in this molecule shows up at about 190 ppm. A C-O single bond lies between 55 and 90 ppm and can be found for piperonal at about 78 ppm. The remaining carbons in the piperonal molecule are part of a benzene ring.
Aromatic carbons have a chemical shift of 115 to 150 ppm. As can be seen below, there are numerous peaks that fall in this range. It can be expected that the carbons bonded to the oxygen atoms have a stronger chemical shift due to the electronegativity of the oxygen.
The peaks for these two carbons could fall in the 145 to 160 ppm range. The carbon attached to the carbonyl group would also have a greater chemical shift, and could be estimated to be in a range from 120 to 135 ppm.
MASS
No comments:
Post a Comment