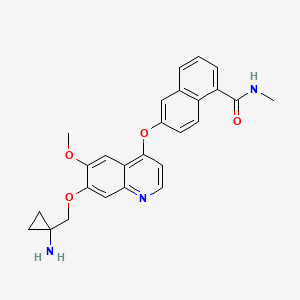
LUCITANIB
6-[7-[(1-aminocyclopropyl)methoxy]-6-methoxyquinolin-4-yl]oxy-N-methylnaphthalene-1-carboxamide
6-(7-((l-aminocyclopropyl)methoxy)-6-methoxyquinolin-4-yloxy)- N-methyl- 1 -naphthamide
1058137-23-7 (E-3810 free base); 1058137-84-0 (E-3810 HCl salt)
E-3810, E-3810 amine, UNII-PP449XA4BH, E3810, Lucitanib [INN], AL3810
http://www.google.com/patents/WO2010105761A1?cl=en
Example 7: Preparation of 6-(7-((l-aminocyclopropyl)methoxy)-6- methoxyquinolin-4-yloxy)-N-methyl-l-naphthamide (I)
A mixture of the compound of Example 6 (0.24 g, 0.42 mmol) in 2 ml of a solution of 40% HBr in acetic acid was stirred at 300C for 3h, then added with 10 ml of water and the reaction mixture was extracted with AcOEt (2 x 10 mL). The organic phases were removed. The aqueous solution was dropwise added with a solution of 50% NaOH to reach pH 10. The mixture was extracted with DCM (3 x 20 mL) and the combined organic phases were dried and evaporated to give a crude containing 6-(7-((l-aminocyclopropyl)methoxy)-6-methoxyquinolin-4- yloxy)-N-methyl-l-naphthamide (I) with purity higher than >94% by LC-MS analysis. This crude was further purified by chromatography on a silica gel column eluting with DCM/MeOH 10: 1), to afford 6-(7-((l- aminocyclopropyl)methoxy)-6-methoxyquinolin-4-yloxy)-N-methyl-l- naphthamide (I) having purity higher than 98% by LC-MS analysis (140 mg, yield: 76%).
1H-NMR (500 MHz, DMSO-d6) δ ppm: 8.47 (d, 2 H), 7.87 (d, 1 H), 7.53 (m, 3 H), 7.51 (m, 1 H), 7.44 (d, 1 H), 7.38 (s, 1 H), 6.50 (d, 1 H), 6.16 (d, 1 H), 5.01 (s, 2 H), 4.05 (s, 2 H), 4.03 (s, 3 H), 3.12 (d, 3 H), 2.09 (m, 2 H), 0.80 (m, 4 H).
LC-MS: M+H+: 444.0
No comments:
Post a Comment